Protein primary structure
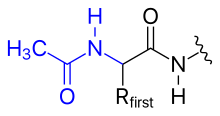
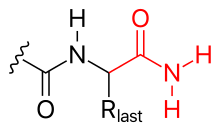
In general, polypeptides are unbranched polymers, so their primary structure can often be specified by the sequence of amino acids along their backbone.
Many other chemical reactions (e.g., cyanylation) have been applied to proteins by chemists, although they are not found in biological systems.
In addition to those listed above, the most important modification of primary structure is peptide cleavage (by chemical hydrolysis or by proteases).
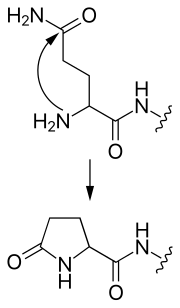
Typically, the hydroxyl group of a serine (rarely, threonine) or the thiol group of a cysteine residue will attack the carbonyl carbon of the preceding peptide bond, forming a tetrahedrally bonded intermediate [classified as a hydroxyoxazolidine (Ser/Thr) or hydroxythiazolidine (Cys) intermediate].
The ester/thioester bond can be resolved in several ways: The compression of amino acid sequences is a comparatively challenging task.
For example, modeling inversions is harder because of the reverse information loss (from amino acids to DNA sequence).
Franz Hofmeister made the proposal in the morning, based on his observations of the biuret reaction in proteins.
Hofmeister was followed a few hours later by Emil Fischer, who had amassed a wealth of chemical details supporting the peptide-bond model.
For completeness, the proposal that proteins contained amide linkages was made as early as 1882 by the French chemist E.
Hermann Staudinger faced similar prejudices in the 1920s when he argued that rubber was composed of macromolecules.
Although never given much credence, these alternative models were finally disproved when Frederick Sanger successfully sequenced insulin[when?]
Protein sequence can be used to predict local features, such as segments of secondary structure, or trans-membrane regions.
If the full-length protein sequence is available, it is possible to estimate its general biophysical properties, such as its isoelectric point.