Phosphorus trioxide
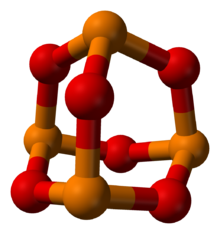
Phosphorus trioxide is the chemical compound with the molecular formula P4O6.
Although the molecular formula suggests the name tetraphosphorus hexoxide, the name phosphorus trioxide preceded the knowledge of the compound's molecular structure, and its usage continues today.
A white solid that melts at room temperature, it is waxy, crystalline and highly toxic, with garlic odor.
[1] It is obtained by the combustion of phosphorus in a limited supply of air at low temperatures.
In a disproportionation reaction, P4O6 is converted into the mixed P(III)P(V) species P4O8 when heated in a sealed tube at 710 K, with the side product being red phosphorus.