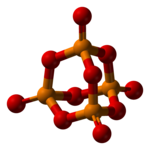
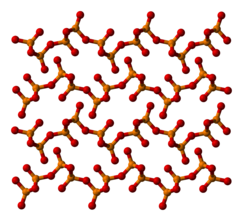
Phosphorus pentoxide
This form can be made by condensing the vapor of phosphorus pentoxide rapidly, and the result is an extremely hygroscopic solid.
[4] The other polymorphs are polymeric, but in each case the phosphorus atoms are bound by a tetrahedron of oxygen atoms, one of which forms a terminal P=O bond involving the donation of the terminal oxygen p-orbital electrons to the antibonding phosphorus-oxygen single bonds.
Phosphorus pentoxide is a potent dehydrating agent as indicated by the exothermic nature of its hydrolysis producing phosphoric acid: However, its utility for drying is limited somewhat by its tendency to form a protective viscous coating that inhibits further dehydration by unspent material.
The most important application is for the conversion of primary amides into nitriles:[7] The indicated coproduct P4O9(OH)2 is an idealized formula for undefined products resulting from the hydration of P4O10.
Alternatively, when combined with a carboxylic acid, the result is the corresponding anhydride:[8] The "Onodera reagent", a solution of P4O10 in DMSO, is employed for the oxidation of alcohols.
Just like sulfur trioxide, it reacts vigorously with water and water-containing substances like wood or cotton, liberates much heat and may even cause fire due to the highly exothermic nature of such reactions.
It is corrosive to metal and is very irritating – it may cause severe burns to the eye, skin, mucous membrane, and respiratory tract even at concentrations as low as 1 mg/m3.