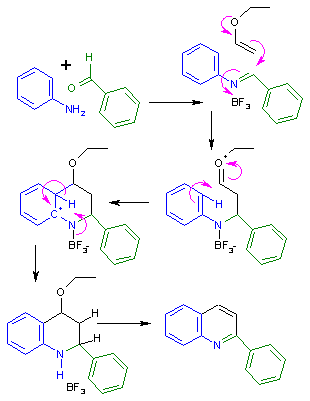
Povarov reaction
In step one aniline and benzaldehyde react to the Schiff base in a condensation reaction.
Two additional elimination reactions create the quinoline ring structure.
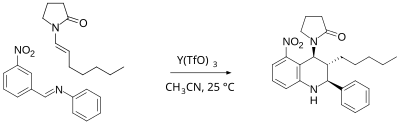
This is in contrast to traditional Diels–Alder reactions, which are stereospecific based on the alkene geometry.
In 2013, Doyle and coworkers reported a Povarov-type, formal [4+2]-cycloaddition reaction between donor-acceptor cyclopropenes and imines (Scheme 3).
Scheme 4 depicts this 4 component reaction with the ethyl ester of glyoxylic acid, 3,4-dihydro-2H-pyran, aniline and ethanol with lewis acid scandium(III) triflate and molecular sieves.