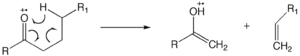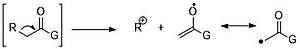Mass spectral interpretation
Due to the high vacuum pressure in the ionization chamber, the mean free path of molecules are varying from 10 cm to 1 km and then the fragmentations are unimolecular processes.
Examining organic compounds, the relative intensity of the molecular ion peak diminishes with branching and with increasing mass in a homologous series.
These peaks result from ions with lifetimes shorter than the time needed to traverse the distance between ionization chamber and the detector.
Several theories can be utilized to predict the fragmentation process, such as the electron octet rule, the resonance stabilization and hyperconjugation and so on.
On addition of heteroatoms, the molecular formula is adjusted by the equivalent mass of carbon and hydrogen.
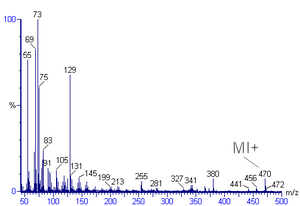
The mass spectrum of methylbromide has two prominent peaks of equal intensity at m/z 94 (M) and 96 (M+2) and then two more at 79 and 81 belonging to the bromine fragment.
The fragmentation pattern of the spectra beside the determination of the molar weight of an unknown compound also suitable to give structural information, especially in combination with the calculation of the degree of unsaturation from the molecular formula (when available).
Neutral fragments frequently lost are carbon monoxide, ethylene, water, ammonia, and hydrogen sulfide.
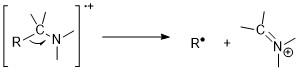
The driving forces for such reaction is the electron donating abilities of the radical sites: N > S, O,π > Cl, Br > H.[11] An example is the cleavage of carbon-carbon bonds next to a heteroatom.
[11] The McLafferty rearrangement can occur in a molecule containing a keto-group and involves β-cleavage, with the gain of the γ-hydrogen atom.
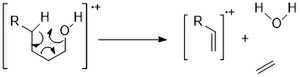
[12][13][14] Ion-neutral complex formation involves bond homolysis or bond heterolysis, in which the fragments do not have enough kinetic energy to separate and, instead, reaction with one another like an ion-molecule reaction.The “1,5 ” hydrogen shift cause transfer of one γ- hydrogen to a radical site on a saturated heteroatom.
Such rearrangement initiates charge-site reaction, resulting in the formation of an odd electron ion and a small neutral molecule ( water, or acid and so on).
The same requirements for The “1,5 ” hydrogen shift occur between proper substituents in the ortho positions of the aromatic rings.
Such rearrangement initiates charge-site reaction, resulting in the formation of an odd electron ion and a small neutral molecule ( water, or HCl and so on).
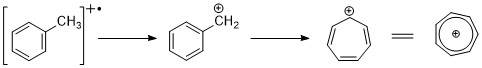
When alkyl groups are attached to the ring, a favorable mode of cleavage is to lose a H-radical to form the tropylium cation (m/z 91).
[2][11] Alkyl substituted benzenes can fragment via the kinetic controlled process to form C6H5+, C6H6+ ions.
[11] Another common mode of fragmentation is the McLafferty rearrangement, which requires the alkyl chain length to be at least longer than 3 carbons.
[11] Alcohols generally have weak molecular ion peaks due to the strong electronegativity of oxygen.
[2][11] Ethers produce slightly more intense molecular ion peaks compared to the corresponding alcohols or alkanes.
Aromatic ethers can generate the C6H5O+ ion by loss of the alkyl group rather than H; this can expel CO as in the phenolic degradation.
[11] There are five types of carbonyl compounds, including aldehydes, ketones, carboxylic acids and esters.
[2] The principal fragmentation modes are described as follows: Alpha-cleavage can occur on either side of the carbonyl functional group since an oxygen lone pair can stabilize the positive charge.
β-cleavage is a characteristic mode of carbonyl compounds' fragmentation due to the resonance stabilization.
For longer chain carbonyl compounds (carbon number is bigger than 4), McLafferty rearrangements are dominant.
According to these fragmentation patterns, the characteristic peaks of carbonyl compounds are summarized in the following table.
The principle fragmentation mode is the loss of an H-atom (M – 1) from the carbon next to the CN group due to the resonance stabilization.