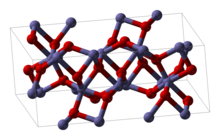
Rhodium(III) oxide
It is a gray solid that is insoluble in ordinary solvents.
The hexagonal form adopts the corundum structure.
[1] Rhodium oxide can be produced via several routes: Rhodium oxide films behave as a fast two-color electrochromic system: Reversible yellow ↔ dark green or yellow ↔ brown-purple color changes are obtained in KOH solutions by applying voltage ~1 V.[7] Rhodium oxide films are transparent and conductive, like indium tin oxide (ITO) - the common transparent electrode, but Rh2O3 has 0.2 eV lower work function than ITO.
Consequently, deposition of rhodium oxide on ITO improves the carrier injection from ITO thereby improving the electrical properties of organic light-emitting diodes.
[5] Rhodium oxides are catalysts for hydroformylation of alkenes,[8] N2O production from NO,[9] and the hydrogenation of CO.[10]