Silicon monoxide
[4] The first precise report on the formation of SiO was in 1887[5] by the chemist Charles F. Maybery (1850–1927) at the Case School of Applied Science in Cleveland.
Maybery claimed that SiO formed as an amorphous greenish-yellow substance with a vitreous luster when silica was reduced with charcoal in the absence of metals in an electric furnace.
[12] Silica itself, or refractories containing SiO2, can be reduced with H2 or CO at high temperatures, e.g.:[13] As the SiO product volatilizes off (is removed), the equilibrium shifts to the right, resulting in the continued consumption of SiO2.
[14][15] Silicon monoxide molecules have been trapped in an argon matrix cooled by helium.
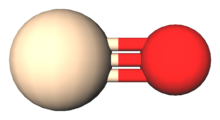
The SiO double bond structure is, notably, an exception to Lewis' octet rule for molecules composed of the light main group elements, whereas the SiO triple bond satisfies this rule.
When metal atoms (such as Na, Al, Pd, Ag, and Au) are co-deposited with SiO, triatomic molecules are produced with linear (AlSiO and PdSiO), non-linear (AgSiO and AuSiO), and ring (NaSiO) structures.
[3] Potter reported SiO solid as yellowish-brown in color and as being an electrical and thermal insulator.
Even though Potter reported the heat of combustion of SiO to be 200 to 800 calories higher than that of an equilibrium mixture of Si and SiO2 (which could, arguably, be used as evidence that SiO is a unique chemical compound),[18] some studies characterized commercially available solid silicon monoxide materials as an inhomogeneous mixture of amorphous SiO2 and amorphous Si with some chemical bonding at the interface of the Si and SiO2 phases.