Silver(I,III) oxide
Ag2O.Ag2O3 Silver(I,III) oxide or tetrasilver tetroxide is the inorganic compound with the formula Ag4O4.
[2] It is a dark brown solid that decomposes with evolution of O2 in water.
It dissolves in concentrated nitric acid to give brown solutions containing the Ag2+ ion.
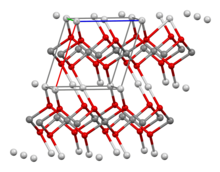
X-ray diffraction studies show that the silver atoms adopt two different coordination environments, one having two collinear oxide neighbours and the other four coplanar oxide neighbours.
In 2010, the FDA issued a warning letter to an American company concerning the firm's marketing of Tetrasil and Genisil ointments of tetrasilver tetroxide for herpes and similar conditions.