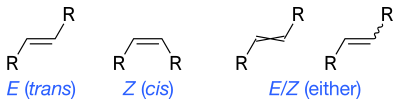Structural formula
Unlike other chemical formula types,[a] which have a limited number of symbols and are capable of only limited descriptive power, structural formulas provide a more complete geometric representation of the molecular structure.
A dashed wedge indicates that the atom is behind the molecule; it is pointing below the plane of the paper.
[4] Wavy single bonds represent unknown or unspecified stereochemistry or a mixture of isomers.
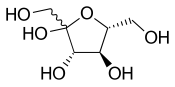
For example, the adjacent diagram shows the fructose molecule with a wavy bond to the HOCH2- group at the left.
[citation needed] Skeletal formulas can depict cis and trans isomers of alkenes.
Wavy single bonds are the standard way to represent unknown or unspecified stereochemistry or a mixture of isomers (as with tetrahedral stereocenters).
Two or three parallel lines between pairs of atoms represent double or triple bonds, respectively.
In addition, all non-bonded electrons (paired or unpaired) and any formal charges on atoms are indicated.
Through the use of Lewis structures, the placement of electrons, whether it is in a bond or in lone pairs, will allow for the identification of the formal charges of the atoms in the molecule to understand the stability and determine the most likely molecule (based on molecular geometry difference) that would be formed in a reaction.
In early organic-chemistry publications, where use of graphics was strongly limited, a typographic system arose to describe organic structures in a line of text.
There are different ways to show the various functional groups in the condensed formulas such as aldehyde as CHO, carboxylic acids as CO2H or COOH, esters as CO2R or COOR.
The sawhorse projection is very similar to a skeletal formula, and it can even use wedges instead of lines to indicate the stereochemistry of the molecule.
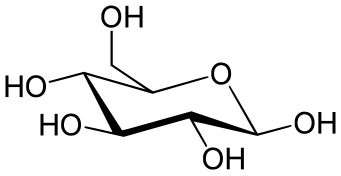
For example, the standard chair conformation of cyclohexane involves a perspective view from slightly above the average plane of the carbon atoms and indicates clearly which groups are axial (pointing vertically up or down) and which are equatorial (almost horizontal, slightly slanted up or down).
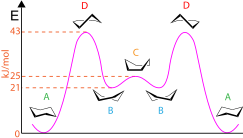
The cyclohexane conformations may also be used to show the potential energy present at each stage as shown in the diagram.
In addition, cyclohexane conformations can be used to indicate if the molecule has any 1,3 diaxial-interactions which are steric interactions between axial substituents on the 1,3, and 5 carbons.
However, an important thing to keep in mind while reading an Haworth projection is that the ring structures are not flat.
Sir Norman Haworth, was a British Chemist, who won a Nobel Prize for his work on Carbohydrates and discovering the structure of Vitamin C. During his discovery, he also deducted different structural formulas which are now referred to as Haworth Projections.
Nonetheless, the Fischer projection is a simple way of depicting multiple sequential stereocenters that does not require or imply any knowledge of actual conformation.
A Fischer projection will restrict a 3-D molecule to 2-D, and therefore, there are limitations to changing the configuration of the chiral centers.
Fischer projections are used to determine the R and S configuration on a chiral carbon and it is done using the Cahn Ingold Prelog rules.
For all dynamic effects, temperature will affect the inter-conversion rates and may change how the structure should be represented.