Monosaccharide
Monosaccharides are the building blocks of disaccharides (such as sucrose, lactose and maltose) and polysaccharides (such as cellulose and starch).
The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two monosaccharides D-glucose and D-fructose.
[1] Monosaccharides can be classified by the number x of carbon atoms they contain: triose (3), tetrose (4), pentose (5), hexose (6), heptose (7), and so on.
A more general nomenclature for open-chain monosaccharides combines a Greek prefix to indicate the number of carbons (tri-, tetr-, pent-, hex-, etc.)
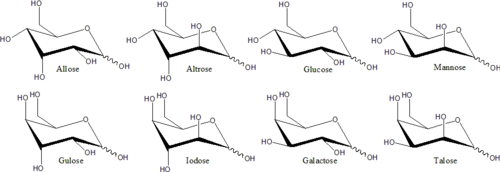
Two monosaccharides with equivalent molecular graphs (same chain length and same carbonyl position) may still be distinct stereoisomers, whose molecules differ in spatial orientation.
This happens only if the molecule contains a stereogenic center, specifically a carbon atom that is chiral (connected to four distinct molecular sub-structures).
The other triose, the aldose H(C=O)(CHOH)2H (glyceraldehyde), has one chiral carbon—the central one, number 2—which is bonded to groups −H, −OH, −C(OH)H2, and −(C=O)H. Therefore, it exists as two stereoisomers whose molecules are mirror images of each other (like a left and a right glove).
The Fischer projection is a systematic way of drawing the skeletal formula of an acyclic monosaccharide so that the handedness of each chiral carbon is well specified.
In the Fischer projection, two mirror-image isomers differ by having the positions of all chiral hydroxyls reversed right-to-left.
Mirror-image isomers are chemically identical in non-chiral environments, but usually have very different biochemical properties and occurrences in nature.
These specific monosaccharide names have conventional three-letter abbreviations, like "Glu" for glucose and "Thr" for threose.
Note that the D- and L- prefixes do not indicate the direction of rotation of polarized light, which is a combined effect of the arrangement at all chiral centers.
The resulting molecule has a hemiacetal or hemiketal group, depending on whether the linear form was an aldose or a ketose.
These forms are called furanoses and pyranoses, respectively—by analogy with furan and pyran, the simplest compounds with the same carbon-oxygen ring (although they lack the double bonds of these two molecules).
For many monosaccharides (including glucose), the cyclic forms predominate, in the solid state and in solutions, and therefore the same name commonly is used for the open- and closed-chain isomers.
The −OH group that replaces the carbonyl's oxygen may end up in two distinct positions relative to the ring's midplane.
Thus each open-chain monosaccharide yields two cyclic isomers (anomers), denoted by the prefixes α- and β-.
The molecule can change between these two forms by a process called mutarotation, that consists in a reversal of the ring-forming reaction followed by another ring formation.