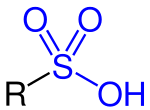
Sulfonic acid
A large scale application of this method is the production of alkylbenzenesulfonic acids: In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile.
Alkylsulfonic acids can be prepared by sulfoxidation whereby alkanes are irradiated with a mixture of sulfur dioxide and oxygen.
Vinylsulfonic acid is derived by hydrolysis of carbyl sulfate, (C2H4(SO3)2), which in turn is obtained by the addition of sulfur trioxide to ethylene.
[6] This property implies an acidity within two or three orders of magnitude of that of HCl(g), whose pKa was recently accurately determined (pKaaq = −5.9).
[citation needed] Because of their polarity, sulfonic acids tend to be crystalline solids or viscous, high-boiling liquids.
Dowex resin are sulfonic acid derivatives of polystyrene and is used as catalysts and for ion exchange (water softening).
Nafion, a fluorinated polymeric sulfonic acid is a component of proton exchange membranes in fuel cells.
Sulfonic esters such as methyl triflate are considered good alkylating agents in organic synthesis.
Sulfonyl fluorides can be produced by treating sulfonic acids with sulfur tetrafluoride:[14] Although strong, the (aryl)C−SO3− bond can be broken by nucleophilic reagents.
Of historic and continuing significance is the α-sulfonation of anthroquinone followed by displacement of the sulfonate group by other nucleophiles, which cannot be installed directly.
[9] An early method for producing phenol involved the base hydrolysis of sodium benzenesulfonate, which can be generated readily from benzene.
[15] The conditions for this reaction are harsh, however, requiring 'fused alkali' or molten sodium hydroxide at 350 °C for benzenesulfonic acid itself.
[17] Sulfonic acids with electron-withdrawing groups (e.g., with NO2 or CN substituents) undergo this transformation much more readily.
Arylsulfonic acids react with two equiv of butyl lithium to give the ortho-lithio derivatives, i.e. ortho-lithiation.