Tin(II) oxide
[4] Metastable, red SnO can be prepared by gentle heating of the precipitate produced by the action of aqueous ammonia on a tin(II) salt.
[5][6] Tin(II) oxide burns in air with a dim green flame to form SnO2.
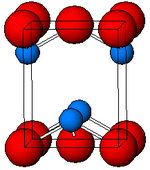
[10] Black, α-SnO adopts the tetragonal PbO layer structure containing four coordinate square pyramidal tin atoms.
[16] The dominant use of stannous oxide is as a precursor in manufacturing of other, typically divalent, tin compounds or salts.
Stannous oxide may also be employed as a reducing agent and in the creation of ruby glass.