Transmembrane protein
The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot.
All beta-barrel transmembrane proteins have simplest up-and-down topology, which may reflect their common evolutionary origin and similar folding mechanism.
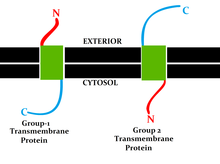
[9] This classification refers to the position of the protein N- and C-termini on the different sides of the lipid bilayer.
[citation needed] Membrane protein structures can be determined by X-ray crystallography, electron microscopy or NMR spectroscopy.
The unfolded state of membrane proteins in detergent micelles is different from that in the thermal denaturation experiments.
[citation needed] This state represents a combination of folded hydrophobic α-helices and partially unfolded segments covered by the detergent.
For example, the "unfolded" bacteriorhodopsin in SDS micelles has four transmembrane α-helices folded, while the rest of the protein is situated at the micelle-water interface and can adopt different types of non-native amphiphilic structures.
Free energy differences between such detergent-denatured and native states are similar to stabilities of water-soluble proteins (< 10 kcal/mol).
If the protein remains unfolded and attached to the translocon for too long, it is degraded by specific "quality control" cellular systems.
It is thought that β-barrel membrane proteins come from one ancestor even having different number of sheets which could be added or doubled during evolution.