VPS35
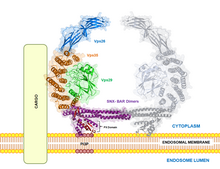
[1][2][3][4][5] VPS35 is part of a complex called the retromer, which is responsible for transporting select cargo proteins between vesicular structures (e.g., endosomes, lysosomes, vacuoles) and the Golgi apparatus.
[3] RNA expression of VPS35 is ubiquitous throughout the body, but are higher in the brain, heart, gonads, spleen, and skeletal muscle, and lower in the lung, liver, kidney, and blood leukocytes.
[5] These mutations also affect many cellular processes overseen by the retromer complex, including vesicular trafficking, plasma membrane receptor recycling, protein aggregation, mitochondrial function, and dopamine signaling.
[10][19] It is characterized by dopaminergic neuron loss in the substantia nigra pars compacta and the toxic aggregation of the α-synuclein protein into Lewy Bodies, all of which cause motor dysfunctions and dopamine deficiency in PD patients.
[7][8] Sequence alignment of VPS35 orthologs between Homo sapiens, Pan troglodytes, Mus musculus, Rattus norvegicus, Bos taurus, Canis familiaris, Gallus gallus, Xenopus laevis, Danio rerio, Drosophila melanogaster, and Saccharomyces cerevisiae has shown that the 620 position is highly mutagenic with a propensity to substitute aspartic acid with asparagine throughout evolution.
[9] Changes in VPS35 affect levels of leucine-rich repeat kinase 2 (LRRK2), a candidate gene involved in PD that helps vesicular trafficking by phosphorylating Rab proteins.
[16] VPS35 overexpression in Drosophila lacking Parkin reverses Parkin-deficient phenotypes, increasing longevity, climbing ability, and decreasing sensitivity to paraquat, a toxic herbicide known to be associated with PD onset in humans.
[16] AD is the most prominent cause of dementia (60-80%) and affects many cognitive abilities in patients, including word retrieval, memory recall, and other general executive functions necessary for basic self-care.
[24] AD pathology typically begins with the formation of amyloid beta (Aβ) plaques in the brain, which trigger an inflammatory response by microglia and cause a cascade of tau accumulation and spreading.
[1] Decreased expression of VPS35 in Drosophila AD models further shows an increase in Aβ plaque formation, BACE1 activity, memory deficits, and synaptic dysfunction.
[6][15] VPS35 deletion in mammalian models of AD is associated with aberrant microglia function and impaired hippocampal development; however, causal variants have yet to be determined.
[2][8][16] Compared to non-mutated VPS35, the VPS35-D620N variant has weaker binding affinity to family with sequence similarity 210 member A (FAM21), a component of the WASH complex, leading to autophagic dysfunction.
[3][9][10] Another result of impaired WASH complex binding is the missorting of Cl-MPR and glucose transporter 1, GLUT1, affecting TGN function and energy utilization.
[6][7][10] Mouse heterozygous knockouts of VPS35 display PD-like phenotypes into adulthood, including α-synuclein aggregation, motor impairments, and a loss of dopaminergic activity in the substantia nigra and striatum.
In rats, overexpression by adeno-associated virus (AAV) of human VPS35-D620N does not alter α-synuclein levels, phosphorylation, or PD pathology in dopaminergic neurons in the substantia nigra.
[1] Mouse knock-in models of VPS35-D620N display no difference in retromer assembly and stability or protein levels related to endolysosomes, autophagy, mitochondria, or α-synuclein in the brain, although there is a reduction in striatal dopamine around 5 months of age.
[15] Inactivity of the retromer complex through VPS35 mutation is thought to degrade Wntless, a membrane protein that regulates Wnt secretion from cells.
[10] Treating mouse hippocampal and cortical neurons with VPS35 small interfering RNA inhibits AMPA receptor trafficking to the dendritic membrane.
[8] Human induced pluripotent stem cell-derived dopaminergic neurons with the VPS35-D620N mutation also shift GluR1 localization away from dendritic spines, altering glutamatergic synaptic transmission.
[3] Mitochondria are organelles that undergo oxidative phosphorylation to produce adenosine triphosphate, or ATP, providing energy for the cell to carry out necessary metabolic and homeostatic processes.
[9] Thiophene thiourea derivatives R33 and R55 have been shown to regulate VPS35 levels, restabilizing the retromer complex and reestablishing endosomal function in AD.
[9] Rapamycin treatment has also been shown to enhance autophagic function and improve clearance of protein aggregates that pay key roles in PD and AD.
[10] There is potential to modulate VPS35 using viral vectors or genome editing techniques like CRISPR/Cas9, however, given VPS35's ubiquitous role in many homeostatic processes, strict dose control and regional specificity would be necessary to achieve a safe, therapeutic effect.
[9] AAV2 vectors have demonstrated safety and efficacy in clinical trials and may be designed to introduce a high yield of non-mutant VPS35 to patients with neurodegenerative diseases.
[10] Speculative therapeutic applications for VPS35 include developing a biomarker assay that detects lower levels of VPS35 in PD or AD patients and identifying cis-regulatory elements within the VPS35 gene for microRNA therapy to reverse VPS35 deficiency.