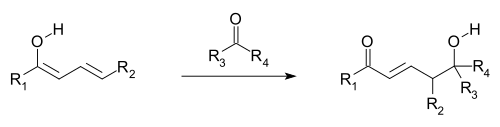
Vinylogy
[1] The concept was introduced in 1926 by Ludwig Claisen to explain the acidic properties of formylacetone and related ketoaldehydes.
Hence, vinylogy is a useful heuristic for the prediction of the behavior of systems that are structurally similar but contain intervening C=C bonds that are conjugated to the attached functional groups.
The insertion of a o- or p-phenylene (i.e., a benzene ring in the 1,2- or 1,4-orientation) also results in some similarities in reactivity (called "phenylogy"), although the effect is generally weaker, as conjugation through the aryl ring requires consideration of resonance forms or intermediates in which aromaticity is disrupted.
Allylic electrophiles often react by vinylogous attack of a nucleophile rather than direct addition.
A further example of vinylogous reactivity: ascorbic acid (Vitamin C) behaves as a vinylogous carboxylic acid by involvement of its carbonyl moiety, a vinyl group within the ring, and the lone pair on the hydroxyl group acting as the conjugated system.