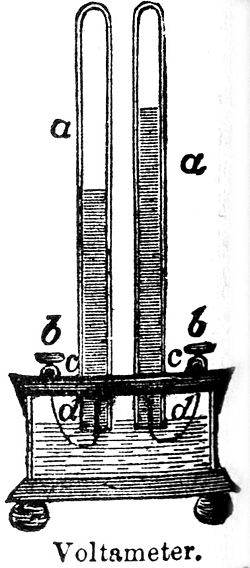
Voltameter
Michael Faraday used an apparatus that he termed a "volta-electrometer"; subsequently John Frederic Daniell called this a "voltameter".
[1] The voltameter is an electrolytic cell and the measurement is made by weighing the element deposited or released at the cathode in a specified time.
In this device, mercury is used to determine the amount of charges transformed during the following reaction: These oxidation/reduction processes have 100% efficiency with the wide range of the current densities.
Measuring of the quantity of electricity (coulombs) is based on the changes of the mass of the mercury electrode.
Mass of the electrode can be increased during cathodic deposition of the mercury ions or decreased during the anodic dissolution of the metal.