Western blot
The secondary antibody is visualized through various methods such as staining, immunofluorescence, and radioactivity, allowing indirect detection of the specific target protein.
[4] The term western blot was given by W. Neal Burnette in 1981,[5] although the method itself was independently invented in 1979 by Jaime Renart, Jakob Reiser, and George Stark at Stanford University,[6] and by Harry Towbin, Theophil Staehelin, and Julian Gordon at the Friedrich Miescher Institute in Basel, Switzerland.
[9] The confirmatory HIV test employs a western blot to detect anti-HIV antibody in a human serum sample.
Recent research utilizing the western blot technique showed an improved detection of EPO in blood and urine based on novel Velum SAR precast horizontal gels optimized for routine analysis.
[19] With the adoption of the horizontal SAR-PAGE in combination with the precast film-supported Velum SAR gels the discriminatory capacity of micro-dose application of rEPO was significantly enhanced.
For medication development, the identification of therapeutic targets, and biological research, it is essential to comprehend where proteins are located within a cell.
[24][3] To achieve efficient protein extraction, a proper homogenization method needs to be chosen due to the fact that it is responsible for bursting the cell membrane and releasing the intracellular components.
SDS-PAGE (SDS-polyacrylamide gel electrophoresis) maintains polypeptides in a denatured state once they have been treated with strong reducing agents to remove secondary and tertiary structure (e.g. disulfide bonds [S-S] to sulfhydryl groups [SH and SH]) and thus allows separation of proteins by their molecular mass.
One lane is usually reserved for a marker or ladder, which is a commercially available mixture of proteins of known molecular weights, typically stained so as to form visible, coloured bands.
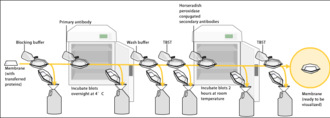
To make the proteins accessible to antibody detection, they are moved from within the gel onto a membrane, a solid support, which is an essential part of the process.
[26] At least seven different approaches for total protein staining have been described for western blot normalization: Ponceau S, stain-free techniques, Sypro Ruby, Epicocconone, Coomassie R-350, Amido Black, and Cy5.
Blocking of non-specific binding is achieved by placing the membrane in a dilute solution of protein – typically 3–5% bovine serum albumin (BSA) or non-fat dry milk (both are inexpensive) in tris-buffered saline (TBS) or I-Block, with a minute percentage (0.1%) of detergent such as Tween 20 or Triton X-100.
This reduces background in the final product of the western blot, leading to clearer results, and eliminates false positives.
For a variety of reasons, this traditionally takes place in a two-step process, although there are now one-step detection methods available for certain applications.
The primary antibodies are generated when a host species or immune cell culture is exposed to the protein of interest (or a part thereof).
Normally, this is part of the immune response, whereas here they are harvested and used as sensitive and specific detection tools that bind the protein directly.
After blocking, a solution of primary antibody (generally between 0.5 and 5 micrograms/mL) diluted in either PBS or TBST wash buffer is incubated with the membrane under gentle agitation for typically an hour at room temperature, or overnight at 4°C.
To allow detection of the target protein, the secondary antibody is commonly linked to biotin or a reporter enzyme such as alkaline phosphatase or horseradish peroxidase.
Therefore, the production of luminescence is proportional to the amount of horseradish peroxidase-conjugated secondary antibody, and therefore, indirectly measures the presence of the target protein.
A sensitive sheet of photographic film is placed against the membrane, and exposure to the light from the reaction creates an image of the antibodies bound to the blot.
This gives researchers and corporations huge advantages in terms of flexibility, reduction of cost, and adds an amplification step to the detection process.
Given the advent of high-throughput protein analysis and lower limits of detection, however, there has been interest in developing one-step probing systems that would allow the process to occur faster and with fewer consumables.
Chemiluminescent detection methods depend on incubation of the western blot with a substrate that will luminesce when exposed to the reporter on the secondary antibody.
The light is then detected by CCD cameras which capture a digital image of the western blot or photographic film.
The image is analysed by densitometry, which evaluates the relative amount of protein staining and quantifies the results in terms of optical density.
The importance of radioactive detections methods is declining due to its hazardous radiation [citation needed], because it is very expensive, health and safety risks are high, and ECL (enhanced chemiluminescence) provides a useful alternative.
[33] In order to ensure that the results of Western blots are reproducible, it is important to report the various parameters mentioned above, including specimen preparation, the concentration of protein used for loading, the percentage of gel and running condition, various transfer methods, attempting to block conditions, the concentration of antibodies, and identification and quantitative determination methods.
Increasing the exposition period in the detection system's software can address weak bands caused by lower sample and antibody concentrations.
Multiple bands might show up in the high molecular weight region because some proteins form dimers, trimers, and multimers; this issue might be solved by heating the sample for longer periods of time.
[3] On account of the presence of these kinds of problems, a variety of improvements are being produced in the fields of preparation of cell lysate and blotting procedures to build up reliable results.