X-inactivation
X-inactivation (also called Lyonization, after English geneticist Mary Lyon) is a process by which one of the copies of the X chromosome is inactivated in therian female mammals.
Unlike the random X-inactivation in placental mammals, inactivation in marsupials applies exclusively to the paternally-derived X chromosome.
In the female germline before meiotic entry, X-inactivation is reversed, so that after meiosis all haploid oocytes contain a single active X chromosome.
This phenomenon, which can be observed in the coloration of tortoiseshell cats when females are heterozygous for the X-linked pigment gene, should not be confused with mosaicism, which is a term that specifically refers to differences in the genotype of various cell populations in the same individual; X-inactivation, which is an epigenetic change that results in a different phenotype, is not a change at the genotypic level.
For an individual cell or lineage the inactivation is therefore skewed or 'non-random', and this can give rise to mild symptoms in female 'carriers' of X-linked genetic disorders.
It is understood that X-chromosome inactivation is a random process, occurring at about the time of gastrulation in the epiblast (cells that will give rise to the embryo).
Preferential inactivation of the paternal X-chromosome occurs in both marsupials and in cell lineages that form the membranes surrounding the embryo,[14] whereas in placental mammals either the maternally or the paternally derived X-chromosome may be inactivated in different cell lines.
For example, a female heterozygous for haemophilia (an X-linked disease) would have about half of her liver cells functioning properly, which is typically enough to ensure normal blood clotting.
The effect of female X heterozygosity is apparent in some localized traits, such as the unique coat pattern of a calico cat.
Females, however, will primarily express the genes or alleles located on the X-chromosomal copy that remains active.
Considering the situation for one gene or multiple genes causing individual differences in a particular phenotype (i.e., causing variation observed in the population for that phenotype), in homozygous females it does not particularly matter which copy of the chromosome is inactivated, as the alleles on both copies are the same.
However, in females that are heterozygous at the causal genes, the inactivation of one copy of the chromosome over the other can have a direct impact on their phenotypic value.
In many cases, heterozygous females may be asymptomatic or only present minor symptoms of a given disorder, such as with X-linked adrenoleukodystrophy.
Typically, each X-chromosome is silenced in half of the cells, but this process is skewed when preferential inactivation of a chromosome occurs.
It is thought that skewing happens either by chance or by a physical characteristic of a chromosome that may cause it to be silenced more or less often, such as an unfavorable mutation.
[23][24] On average, each X chromosome is inactivated in half of the cells, although 5-20% of women display X-inactivation skewing.
A study looking at both symptomatic and asymptomatic females who were heterozygous for Duchenne and Becker muscular dystrophies (DMD) found no apparent link between transcript expression and skewed X-Inactivation.
[27][28] The XIC contains four non-translated RNA genes, Xist, Tsix, Jpx and Ftx, which are involved in X-inactivation.
Rep A inhibits the function of Tsix, the antisense of Xist, in conjunction with eliminating expression of Xite.
[38] The Barr body is generally located on the periphery of the nucleus, is late replicating within the cell cycle, and, as it contains the Xi, contains heterochromatin modifications and the Xist RNA.
Examining normal tissues and tumors from females heterozygous for isoenzymes of the sex-linked G6PD gene demonstrated that tumor cells from such individuals express only one form of G6PD, whereas normal tissues are composed of a nearly equal mixture of cells expressing the two different phenotypes.
[46] Besides, measuring the methylation (inactivation) status of the polymorphic human androgen receptor (HUMARA) located on X-chromosome is considered the most accurate method to assess clonality in female cancer biopsies.
[47] A great variety of tumors was tested by this method, some, such as renal cell carcinoma,[48] found monoclonal while others (e.g. mesothelioma[49]) were reported polyclonal.
In these modified stem cells, the Xist-mediated gene silencing seems to reverse some of the defects associated with Down syndrome.
In 1959 Susumu Ohno showed that the two X chromosomes of mammals were different: one appeared similar to the autosomes; the other was condensed and heterochromatic.


1.Early stage embryonic cell of a female human
2.Maternal X chromosome
3.Paternal X chromosome
4.Mitosis and random X-chromosome inactivation event
5.Paternal chromosome is randomly inactivated in one daughter cell, maternal chromosome is inactivated in the other
6.Paternal chromosome is randomly inactivated in both daughter cells
7.Maternal chromosome is randomly inactivated in both daughter cells
8.Three possible random combination outcomes

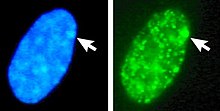
Left: DNA (DAPI)-stained nucleus. Arrow indicates the location of Barr body(Xi). Right: DNA associated histones protein detected
