Xanthine
Xanthine (/ˈzænθiːn/ or /ˈzænθaɪn/, from Ancient Greek ξανθός xanthós 'yellow' for its yellowish-white appearance; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base found in most human body tissues and fluids, as well as in other organisms.
[2] In contrast to other, more potent stimulants like sympathomimetic amines, xanthines mainly act to oppose the actions of adenosine, and increase alertness in the central nervous system.
[2] Methylxanthines (methylated xanthines), which include caffeine, aminophylline, IBMX, paraxanthine, pentoxifylline, theobromine, theophylline, and 7-methylxanthine (heteroxanthine), among others, affect the airways, increase heart rate and force of contraction, and at high concentrations can cause cardiac arrhythmias.
Phosphodiesterase inhibitors raise intracellular cAMP, activate PKA, inhibit TNF-α synthesis,[2][5][4] and leukotriene[6] and reduce inflammation and innate immunity.
[2] Studies reported in 2008, based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, suggested that xanthine and related chemicals, including the RNA component uracil, have been formed extraterrestrially.
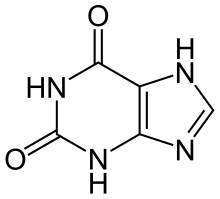



Caffeine: R 1 = R 2 = R 3 = CH 3
Theobromine: R 1 = H, R 2 = R 3 = CH 3
Theophylline: R 1 = R 2 = CH 3 , R 3 = H