Antioxidant
Antioxidants are compounds that inhibit oxidation (usually occurring as autoxidation), a chemical reaction that can produce free radicals.
Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes.
In cells, antioxidants such as glutathione, mycothiol, or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.
[3][4] As part of their adaptation from marine life, terrestrial plants began producing non-marine antioxidants such as ascorbic acid (vitamin C), polyphenols, and tocopherols.
The evolution of angiosperm plants between 50 and 200 million years ago resulted in the development of many antioxidant pigments – particularly during the Jurassic period – as chemical defences against reactive oxygen species that are byproducts of photosynthesis.
In the late 19th and early 20th centuries, extensive study concentrated on the use of antioxidants in important industrial processes, such as the prevention of metal corrosion, the vulcanization of rubber, and the polymerization of fuels in the fouling of internal combustion engines.
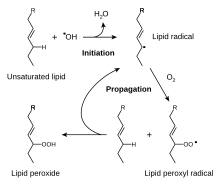
[6] Early research on the role of antioxidants in biology focused on their use in preventing the oxidation of unsaturated fats, which is the cause of rancidity.
Antioxidants are an especially important class of preservatives as, unlike bacterial or fungal spoilage, oxidation reactions still occur relatively rapidly in frozen or refrigerated food.
These molecules undergo free radical chain reactions, but antioxidants inhibit them by preventing the oxidation processes.
For example, phenolic antioxidants such as stilbenes, flavonoids, and hydroxycinnamic acid strongly absorb UV radiation due to the presence of chromophores.
[21] Antioxidants may be added to industrial products, such as stabilizers in fuels and additives in lubricants, to prevent oxidation and polymerization that leads to the formation of engine-fouling residues.
[24] Polymers containing double bonds in their main chains, such as natural rubber and polybutadiene, are especially susceptible to oxidation and ozonolysis.
SPAs are common in indoor dust, small air particles, sediment, sewage, river water and wastewater.
[53] However, although these enzymes can produce oxidants, the relative importance of the electron transfer chain to other processes that generate peroxide is unclear.
[54][55] In plants, algae, and cyanobacteria, reactive oxygen species are also produced during photosynthesis,[56] particularly under conditions of high light intensity.
[43] Some compounds contribute to antioxidant defense by chelating transition metals and preventing them from catalyzing the production of free radicals in the cell.
[81] Ascorbic acid or vitamin C, an oxidation-reduction (redox) catalyst found in both animals and plants,[82] can reduce, and thereby neutralize, reactive oxygen species such as hydrogen peroxide.
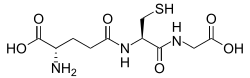
[86] Due to its high concentration and its central role in maintaining the cell's redox state, glutathione is one of the most important cellular antioxidants.
This reaction produces oxidised α-tocopheroxyl radicals that can be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol or ubiquinol.
[100][101] The functions of the other forms of vitamin E are even less well understood, although γ-tocopherol is a nucleophile that may react with electrophilic mutagens,[94] and tocotrienols may be important in protecting neurons from damage.
With the presence of transition metals, there are low concentrations of ascorbic acid that can act as a radical scavenger in the Fenton reaction.
This detoxification pathway is the result of multiple enzymes, with superoxide dismutases catalysing the first step and then catalases and various peroxidases removing hydrogen peroxide.
[109] Superoxide dismutase enzymes contain metal ion cofactors that, depending on the isozyme, can be copper, zinc, manganese or iron.
Surprisingly, glutathione peroxidase 1 is dispensable, as mice lacking this enzyme have normal lifespans,[134] but they are hypersensitive to induced oxidative stress.
[143] Pharmaceuticals and supplements that have antioxidant properties suppress the formation of free radicals by inhibiting oxidation processes.
[144] Relatively strong reducing acids can have antinutrient effects by binding to dietary minerals such as iron and zinc in the gastrointestinal tract and preventing them from being absorbed.
[146] Calcium and iron deficiencies are not uncommon in diets in developing countries where less meat is eaten and there is high consumption of phytic acid from beans and unleavened whole grain bread.
However, germination, soaking, or microbial fermentation are all household strategies that reduce the phytate and polyphenol content of unrefined cereal.
[157][158] Overall, the large number of clinical trials carried out on antioxidant supplements suggest that either these products have no effect on health, or that they cause a small increase in mortality in elderly or vulnerable populations.
[170][171] Earlier measurements and ratings by the United States Department of Agriculture were withdrawn in 2012 as biologically irrelevant to human health, referring to an absence of physiological evidence for polyphenols having antioxidant properties in vivo.