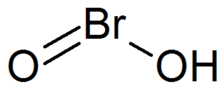Bromous acid
[1] In 1905, Richards A. H. proved the existence of bromous acid through a series of experiments involving silver nitrate (AgNO3) and bromine.
[2] The reaction of excess cold aqueous to form hypobromous acid (HBrO), silver bromide (AgBr) and nitric acid (HNO3): Richards discovered that the effect of adding excess liquid bromine in a concentrated silver nitrate (AgNO3) resulted in a different reaction mechanism.
[2] According to Richards, hypobromous acid (HBrO) arises by the reaction of bromine and silver nitrate solution:[2] The molecule HBrO2 has a bent structure with ∠(H−O−Br) angles of 106.1°.
[8] In comparison to other oxygen-centered oxidants (hypohalites, anions of peroxides) and in line with its low basicity, bromite is a rather weak nucleophile.
[9] Rate constants of bromite towards carbocations and acceptor-substituted olefins are by 1–3 orders of magnitude lower than the ones measured with hypobromite.