Cobalt(II,III) oxide
CoO.Co2O3 Cobalt(II,III) oxide is an inorganic compound with the formula Co3O4.
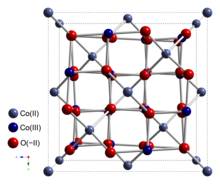
[4] Co3O4 adopts the normal spinel structure, with Co2+ ions in tetrahedral interstices and Co3+ ions in the octahedral interstices of the cubic close-packed lattice of oxide anions.
[4] Cobalt(II) oxide, CoO, converts to Co3O4 upon heating at around 600–700 °C in air.
[4][5] These reactions are described by the following equilibrium: Cobalt(II,III) oxide is used as a blue coloring agent for pottery enamel and glass, as an alternative to cobalt(II) oxide.
Cobalt compounds are potentially poisonous in large amounts.