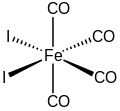
Iron tetracarbonyl diiodide
Iron tetracarbonyl diiodide is the inorganic compound with the formula FeI2(CO)4.
The molecule features four carbonyl ligands and two iodides.
As confirmed by X-ray crystallography, the compound has cis stereochemistry.
[1] It is a black solid that is soluble in dichloromethane and related organic solvents.
It is prepared by the reaction of molecular iodine with iron pentacarbonyl, following a procedure first reported by Hieber and Wirschung in 1940:[2] Iron tetracarbonyl diiodide reacts with a variety of Lewis bases with displacement of one or two CO ligands.