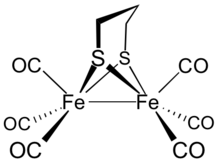
Diiron propanedithiolate hexacarbonyl
Diiron propanedithiolate hexacarbonyl is the organoiron complex with the formula Fe2(S2C3H6)(CO)6.
[1] It adopts a symmetrical structure with six terminal CO ligands.
[3] It is prepared by the reaction of 1,3-propanedithiol with triiron dodecacarbonyl: In general, the CO ligands can be substituted by cyanide, phosphines, isocyanides, N-heterocyclic carbenes, and other donor ligands.
Monosubstitution can be achieved through an in situ generation of the acetonitrile complex.
[4][5] Upon irradiation of Fe2(S2C3H6)(CO)6 with ultraviolet (UV) light, CO-photolysis occurs with the transient formation of the unsaturated species followed by the formation of the solvent adduct.