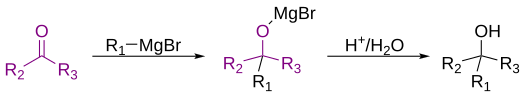
Grignard reaction
In the Merck Index, published online by the Royal Society of Chemistry, the classical definition is acknowledged, followed by "A more modern interpretation extends the scope of the reaction to include the addition of Grignard reagents to a wide variety of electrophilic substrates.
The addition of the Grignard reagent to the carbonyl group typically proceeds through a six-membered ring transition state, as shown below.
[11] A recent computational study suggests that the operative mechanism (polar vs. radical) is substrate-dependent, with the reduction potential of the carbonyl compound serving as a key parameter.
[3] Otherwise, the reaction will fail because the Grignard reagent will act as a base rather than a nucleophile and pick up a labile proton rather than attacking the electrophilic site.
This will result in no formation of the desired product as the R-group of the Grignard reagent will become protonated while the MgX portion will stabilize the deprotonated species.