Holmium(III) oxide
Typically, the oxides of the trivalent lanthanides coexist in nature, and separation of these components requires specialized methods.
The yellow color originates from abundant lattice defects (such as oxygen vacancies) and is related to internal transitions at the Ho3+ ions.
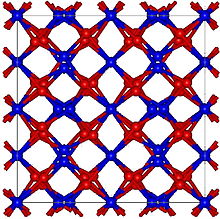
[2] Holmium oxide has a cubic, yet rather complex bixbyite structure, with many atoms per unit cell and a large lattice constant of 1.06 nm.
[6][7] Later in 1878, Per Teodor Cleve independently discovered the element while he was working on erbia earth (erbium oxide).
A typical extraction process of holmium oxide can be simplified as follows: the mineral mixtures are crushed and ground.
After separation, it is treated with hot concentrated sulfuric acid to produce water-soluble sulfates of several rare earth elements.
The rare earth ions are then selectively washed out by suitable complexing agent, such as ammonium citrate or nitrilotriacetate.
This laser is eye safe and is used in medicine, lidars, wind velocity measurements and atmosphere monitoring.
[15] Holmium(III) oxide is, compared to many other compounds, not very dangerous, although repeated overexposure can cause granuloma and hemoglobinemia.