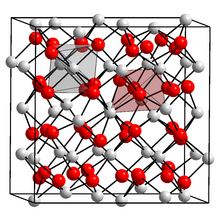
Indium(III) oxide
The rhombohedral phase is produced at high temperatures and pressures or when using non-equilibrium growth methods.
The superconducting transition temperature Tc depends on the doping and film structure and is below 3.3 K.[8] Bulk samples can be prepared by heating indium(III) hydroxide or the nitrate, carbonate or sulfate.
[10] Monocrystalline nanowires can be synthesized from indium oxide by laser ablation, allowing precise diameter control down to 10 nm.
[11] Indium oxide nanowires can serve as sensitive and specific redox protein sensors.
[15] Reacting with a range of metal trioxides produces perovskites[16] for example: Indium oxide is used in some types of batteries, thin film infrared reflectors transparent for visible light (hot mirrors), some optical coatings, and some antistatic coatings.