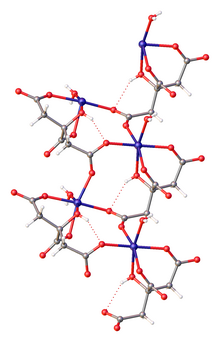
Iron(II) citrate
That complex is the coordination polymer with the formula [Fe(H2O)6]2+{[Fe(C6H5O7)(H2O)]−}2.2H2O, where C6H5O73- is HOC(CH2CO2−)2(CO2−, i.e., the triple conjugate base of citric acid wherein the three carboxylic acid groups are ionized.
[3] Ferrous citrates are all paramagnetic, reflecting the weak crystal field of the carboxylate ligands.
[4] Ferrous citrates are produced by treating disodium citrate Na2C6H6O7 with sources of iron(II) aquo complexes, such as iron(II) sulfate.
It is a nutrient supplement approved by the FDA.
This article about an organic compound is a stub.