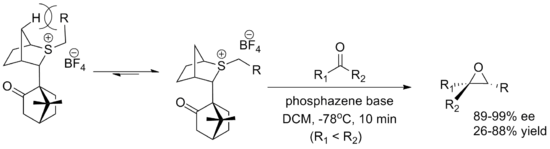Johnson–Corey–Chaykovsky reaction
The reaction involves addition of a sulfur ylide to a ketone, aldehyde, imine, or enone to produce the corresponding 3-membered ring.
A negative charge is transferred to the heteroatom and because the sulfonium cation is a good leaving group it gets expelled forming the ring.
Initial addition of the ylide results in a betaine with adjacent charges; density functional theory calculations have shown that the rate-limiting step is rotation of the central bond into the conformer necessary for backside attack on the sulfonium.
It has seen use in a number of high-profile total syntheses, as detailed below, and is generally recognized as a powerful transformative tool in the organic repertoire.
The substitution pattern can influence the ease of preparation for the reagents (typically from the sulfonium halide, e.g. trimethylsulfonium iodide) and overall reaction rate in various ways.
The development of an enantioselective (i.e. yielding an enantiomeric excess, which is labelled as "ee") variant of the Johnson–Corey–Chaykovsky reaction remains an active area of academic research.
The use of chiral sulfides in a stoichiometric fashion has proved more successful than the corresponding catalytic variants, but the substrate scope is still limited in all cases.
The catalytic variants have been developed almost exclusively for enantioselective purposes; typical organosulfide reagents are not prohibitively expensive and the racemic reactions can be carried out with equimolar amounts of ylide without raising costs significantly.
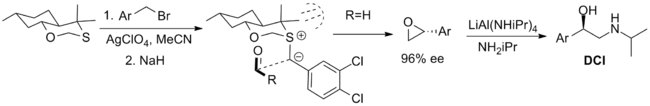
The first is a bicyclic oxathiane that has been employed in the synthesis of the β-adrenergic compound dichloroisoproterenol (DCI) but is limited by the availability of only one enantiomer of the reagent.
The trouble stems from the need for a nucleophilic sulfide that efficiently generates the ylide which can also act as a good leaving group to form the epoxide.
[5][2] Aggarwal has developed an alternative method employing the same sulfide as above and a novel alkylation involving a rhodium carbenoid formed in situ.
The method too has limited substrate scope, failing for any electrophiles possessing basic substituents due to competitive consumption of the carbenoid.







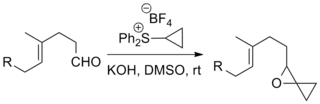





![[4+1] cycloaddition with Corey–Chaykovsky reagent](http://upload.wikimedia.org/wikipedia/commons/thumb/c/cc/CCR41.png/320px-CCR41.png)