Mineralogy
[4] The modern study of mineralogy was founded on the principles of crystallography (the origins of geometric crystallography, itself, can be traced back to the mineralogy practiced in the eighteenth and nineteenth centuries) and to the microscopic study of rock sections with the invention of the microscope in the 17th century.
[5]: 4 More recently, driven by advances in experimental technique (such as neutron diffraction) and available computational power, the latter of which has enabled extremely accurate atomic-scale simulations of the behaviour of crystals, the science has branched out to consider more general problems in the fields of inorganic chemistry and solid-state physics.
In particular, the field has made great advances in the understanding of the relationship between the atomic-scale structure of minerals and their function; in nature, prominent examples would be accurate measurement and prediction of the elastic properties of minerals, which has led to new insight into seismological behaviour of rocks and depth-related discontinuities in seismograms of the Earth's mantle.
An initial step in identifying a mineral is to examine its physical properties, many of which can be measured on a hand sample.
These can be classified into density (often given as specific gravity); measures of mechanical cohesion (hardness, tenacity, cleavage, fracture, parting); macroscopic visual properties (luster, color, streak, luminescence, diaphaneity); magnetic and electric properties; radioactivity and solubility in hydrogen chloride (HCl).
In the Mohs scale, a standard set of minerals are numbered in order of increasing hardness from 1 (talc) to 10 (diamond).
Where these two kinds of break do not occur, fracture is a less orderly form that may be conchoidal (having smooth curves resembling the interior of a shell), fibrous, splintery, hackly (jagged with sharp edges), or uneven.
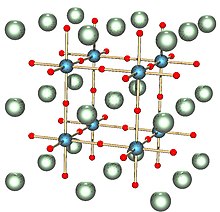
Diffraction, the constructive and destructive interference between waves scattered at different atoms, leads to distinctive patterns of high and low intensity that depend on the geometry of the crystal.
[12] Powder diffraction can distinguish between minerals that may appear the same in a hand sample, for example quartz and its polymorphs tridymite and cristobalite.
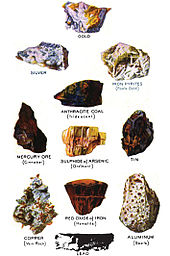
[9]: 150–151 A few minerals are chemical elements, including sulfur, copper, silver, and gold, but the vast majority are compounds.
[9]: 225–226 Other techniques are X-ray fluorescence, electron microprobe analysis atom probe tomography and optical emission spectrography.
Crystals whose point symmetry group falls in the cubic system are isotropic: the index does not depend on direction.
[14] The Manual of Mineralogy places minerals in the following classes: native elements, sulfides, sulfosalts, oxides and hydroxides, halides, carbonates, nitrates and borates, sulfates, chromates, molybdates and tungstates, phosphates, arsenates and vanadates, and silicates.
[9] The environments of mineral formation and growth are highly varied, ranging from slow crystallization at the high temperatures and pressures of igneous melts deep within the Earth's crust to the low temperature precipitation from a saline brine at the Earth's surface.
[22] This database integrates the crowd-sourced site Mindat.org, which has over 690,000 mineral-locality pairs, with the official IMA list of approved minerals and age data from geological publications.
Some factors are deterministic, such as the chemical nature of a mineral and conditions for its stability; but mineralogy can also be affected by the processes that determine a planet's composition.
In a 2015 paper, Robert Hazen and others analyzed the number of minerals involving each element as a function of its abundance.
The model predicts that thousands more mineral species may await discovery or have formed and then been lost to erosion, burial or other processes.
[24][25][26][27] In another use of big data sets, network theory was applied to a dataset of carbon minerals, revealing new patterns in their diversity and distribution.
The analysis can show which minerals tend to coexist and what conditions (geological, physical, chemical and biological) are associated with them.