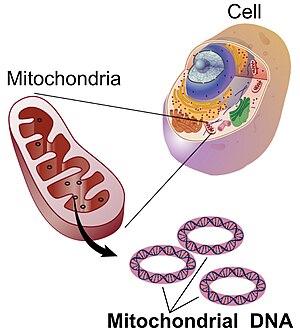
Mitochondrial DNA
Nuclear and mitochondrial DNA are thought to have separate evolutionary origins, with the mtDNA derived from the circular genomes of bacteria engulfed by the ancestors of modern eukaryotic cells.
In the cells of extant organisms, the vast majority of the proteins in the mitochondria (numbering approximately 1500 different types in mammals) are coded by nuclear DNA, but the genes for some, if not most, of them are thought to be of bacterial origin, having been transferred to the eukaryotic nucleus during evolution.
[11] The difficulty of targeting remotely-produced hydrophobic protein products to the mitochondrion is one hypothesis for why some genes are retained in mtDNA;[12] colocalisation for redox regulation is another, citing the desirability of localised control over mitochondrial machinery.
[13] Recent analysis of a wide range of mtDNA genomes suggests that both these features may dictate mitochondrial gene retention.
[19] In February 2020, a jellyfish-related parasite – Henneguya salminicola – was discovered that lacks a mitochondrial genome but retains structures deemed mitochondrion-related organelles.
[27] The genome of the mitochondrion of the cucumber (Cucumis sativus) consists of three circular chromosomes (lengths 1556, 84 and 45 kilobases), which are entirely or largely autonomous with regard to their replication.
[citation needed] The smallest mitochondrial genome sequenced to date is the 5,967 bp mtDNA of the parasite Plasmodium falciparum.
[33] The resulting reduction in per-cell copy number of mtDNA plays a role in the mitochondrial bottleneck, exploiting cell-to-cell variability to ameliorate the inheritance of damaging mutations.
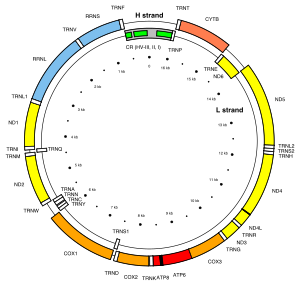
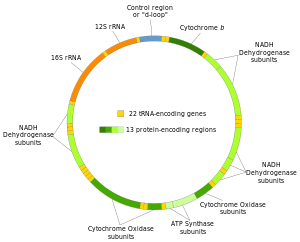
[41] The promoters for the initiation of the transcription of the heavy and light strands are located in the main non-coding region of the mtDNA called the displacement loop, the D-loop.
[42] Interestingly, while the expression of protein-encoding genes was stimulated by ACTH, the levels of the mitochondrial 16S rRNA showed no significant change.
In 1999 it was reported that paternal sperm mitochondria (containing mtDNA) are marked with ubiquitin to select them for later destruction inside the embryo.
These 440 base pairs are compared to the same regions of other individuals (either specific people or subjects in a database) to determine maternal lineage.
[52] The concept of the Mitochondrial Eve is based on the same type of analysis, attempting to discover the origin of humanity by tracking the lineage back in time.
[62][63][64][46] Although many of these cases involve cloned embryos or subsequent rejection of the paternal mitochondria, others document in vivo inheritance and persistence under lab conditions.
[71] The concept that mtDNA is particularly susceptible to reactive oxygen species generated by the respiratory chain due to its proximity remains controversial.
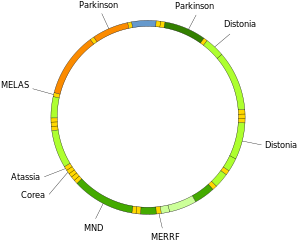
Mutations of mitochondrial DNA can lead to a number of illnesses including exercise intolerance and Kearns–Sayre syndrome (KSS), which causes a person to lose full function of heart, eye, and muscle movements.
The within-cell and between-cell distributions of heteroplasmy dictate the onset and severity of disease[79] and are influenced by complicated stochastic processes within the cell and during development.
[88] However, this concept was conclusively disproved when it was demonstrated that mice, which were genetically altered to accumulate mtDNA mutations at accelerated rate do age prematurely, but their tissues do not produce more ROS as predicted by the 'Vicious Cycle' hypothesis.
[90] The application of a mitochondrial-specific ROS scavenger, which lead to a significant longevity of the mice studied,[91] suggests that mitochondria may still be well-implicated in ageing.
[94] In a recent study, it was shown that dietary restriction can reverse ageing alterations by affecting the accumulation of mtDNA damage in several organs of rats.
[97] Analysis of the brains of AD patients suggested an impaired function of the DNA repair pathway, which would cause reduce the overall quality of mtDNA.
[99] Mutant huntingtin protein promotes oxidative damage to mtDNA, as well as nuclear DNA, that may contribute to Huntington's disease pathology.
In persons with amyotrophic lateral sclerosis (ALS), the enzymes that normally repair 8-oxoG DNA damages in the mtDNA of spinal motor neurons are impaired.
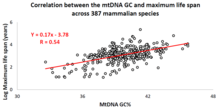
[citation needed] Over the past decade, an Israeli research group led by Professor Vadim Fraifeld has shown that strong and significant correlations exist between the mtDNA base composition and animal species-specific maximum life spans.
[102][103][104] As demonstrated in their work, higher mtDNA guanine + cytosine content (GC%) strongly associates with longer maximum life spans across animal species.
[103] To support the scientific community in carrying out comparative analyses between mtDNA features and longevity across animals, a dedicated database was built named MitoAge.
An association between mtDNA mutational spectrum and species-specific life-history traits in mammals opens a possibility to link these factors together discovering new life-history-specific mutagens in different groups of organisms.
[citation needed] Deletion breakpoints frequently occur within or near regions showing non-canonical (non-B) conformations, namely hairpins, cruciforms and cloverleaf-like elements.
In addition, higher breakpoint densities were consistently observed within GC-skewed regions and in the close vicinity of the degenerate sequence motif YMMYMNNMMHM.
[116] Mitochondrial DNA was first admitted into evidence in California, United States, in the successful prosecution of David Westerfield for the 2002 kidnapping and murder of 7-year-old Danielle van Dam in San Diego: it was used for both human and dog identification.