Naturally occurring phenols
[4] Organisms sometimes synthesize phenolic compounds in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding.
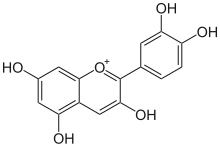
A diverse family natural phenols are the flavonoids, which include several thousand compounds, among them the flavonols, flavones, flavan-3ol (catechins), flavanones, anthocyanidins, and isoflavonoids.
Two natural phenols from two different categories, for instance a flavonoid and a lignan, can combine to form a hybrid class like the flavonolignans.
Nomenclature of polymers: Plants in the genus Humulus and Cannabis produce terpenophenolic metabolites, compounds that are meroterpenes.
According to Woodward's rules, bathochromic shifts often also happen suggesting the presence of delocalised π electrons arising from a conjugation between the benzene and vinyls groups.
[14] As molecules with higher conjugation levels undergo this bathochromic shift phenomenon, a part of the visible spectrum is absorbed.
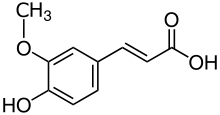
Acylation with cinnamic acids of anthocyanidins shifted color tonality (CIE Lab hue angle) to purple.
[15] Here is a series of UV visible spectra of molecules classified from left to right according to their conjugation level:[citation needed] The absorbance pattern responsible for the red color of anthocyanins may be complementary to that of green chlorophyll in photosynthetically active tissues such as young Quercus coccifera leaves.
The white rot fungus Phanerochaete chrysosporium can remove up to 80% of phenolic compounds from coking waste water.
Additionally, derivatives have been made of phenolic compound, combretastatin A-4, an anticancer molecule, including nitrogen or halogens atoms to increase the efficacy of the treatment.
After proper mixing of the sample and the reagent, the mixture is incubated for 10 minutes at ambient temperature and the absorbance of the solution is read at 440 nm.
[34] Quantitation results produced by the means of diode array detector-coupled HPLC are generally given as relative rather than absolute values as there is a lack of commercially available standards for every phenolic molecules.
The method measures the ability of compounds to prevent the formation of DCF by 2,2'-Azobis(2-amidinopropane) dihydrochloride (ABAP)-generated peroxyl radicals in human hepatocarcinoma HepG2 cells.
[38] Larvae of the model animal Galleria mellonella, also called waxworms, can be used to test the antioxidant effect of individual molecules using boric acid in food to induce an oxidative stress.
are the subject of research into the natural production of butylated hydroxytoluene (BHT),[43] an antioxidant, food additive and industrial chemical.
[50] The green alga Botryococcus braunii is the subject of research into the natural production of butylated hydroxytoluene (BHT),[43] an antioxidant, food additive and industrial chemical.
[61] The hardening of the protein component of insect cuticle has been shown to be due to the tanning action of an agent produced by oxidation of a phenolic substance forming sclerotin.
[citation needed] Decomposition of dead plant material causes complex organic compounds to be slowly oxidized lignin-like humus or to break down into simpler forms (sugars and amino sugars, aliphatic and phenolic organic acids), which are further transformed into microbial biomass (microbial humus) or are reorganized, and further oxidized, into humic assemblages (fulvic and humic acids), which bind to clay minerals and metal hydroxides.
[citation needed] There has been a long debate about the ability of plants to uptake humic substances from their root systems and to metabolize them.
They might be degraded and mineralized as a carbon source by heterotrophic microorganisms; they can be transformed into insoluble and recalcitrant humic substances by polymerization and condensation reactions (with the contribution of soil organisms); they might adsorb to clay minerals or form chelates with aluminium or iron ions; or they might remain in dissolved form, leached by percolating water, and finally leave the ecosystem as part of dissolved organic carbon (DOC).
[4] Leaching is the process by which cations such as iron (Fe) and aluminum (Al), as well as organic matter, are removed from the litterfall and transported downward into the soil below.
This process is known as podzolization and is particularly intense in boreal and cool temperate forests that are mainly constituted by coniferous pines, whose litterfall is rich in phenolic compounds and fulvic acid.
[77] Phenolic compounds can act as protective agents, inhibitors, natural animal toxicants and pesticides against invading organisms, i.e. herbivores, nematodes, phytophagous insects, and fungal and bacterial pathogens.
[79] In tropical Sargassum and Turbinaria species that are often preferentially consumed by herbivorous fishes and echinoids, there is a relatively low level of phenolics and tannins.
These compounds are typically found from wounded plants, and as a result VirA is used by Agrobacterium tumefaciens to locate potential host organisms for infection.
[citation needed] The aquatic vascular plant Myriophyllum spicatum produces ellagic, gallic and pyrogallic acids and (+)-catechin, allelopathic phenolic compounds inhibiting the growth of blue-green alga Microcystis aeruginosa.
[97] Acetosyringone has been best known for its involvement in plant-pathogen recognition,[98] especially its role as a signal attracting and transforming unique, oncogenic bacteria in genus Agrobacterium.
[100] Notable sources of natural phenols in human nutrition include berries, tea, beer, olive oil, chocolate or cocoa, coffee, pomegranates, popcorn, yerba maté, fruits and fruit based drinks (including cider, wine and vinegar) and vegetables.
Phenolic compounds, when used in beverages, such as prune juice, have been shown to be helpful in the color and sensory components, such as alleviating bitterness.
In subsequent phase II reactions, these activated metabolites are conjugated with charged species such as glutathione, sulfate, glycine or glucuronic acid.