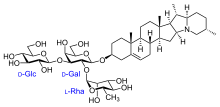
Saponin
They are present in a wide range of plant species throughout the bark, leaves, stems, roots and flowers but particularly in soapwort (genus Saponaria), a flowering plant, the soapbark tree (Quillaja saponaria), common corn-cockle (Agrostemma githago L.), baby's breath (Gypsophila spp.)
They are used in soaps, medicines (e.g. drug adjuvants), fire extinguishers, dietary supplements, steroid synthesis, and in carbonated beverages (for example, being responsible for maintaining the head on root beer).
Triterpene glycosides exhibit a wide range of biological activities and pharmacological properties, making them valuable in traditional medicine and modern drug discovery.
[10] Quillaja is toxic when consumed in large amounts, involving possible liver damage, gastric pain, diarrhea, or other adverse effects.
Within these families, this class of chemical compounds is found in various parts of the plant: leaves, stems, roots, bulbs, blossom and fruit.
[18] Commercial formulations of plant-derived saponins, e.g., from the soap bark tree, Quillaja saponaria, and those from other sources are available via controlled manufacturing processes, which make them of use as chemical and biomedical reagents.
[19] Soyasaponins are a group of structurally complex oleanane-type triterpenoid saponins that include soyasapogenol (aglycone) and oligosaccharide moieties biosynthesized on soybean tissues.
[citation needed] Some plant saponins (e.g., from oat and spinach) may enhance nutrient absorption and aid in animal digestion.
However, saponins are often bitter to taste, and so can reduce plant palatability (e.g., in livestock feeds), or even imbue them with life-threatening animal toxicity.
Further research is needed to define the roles of these natural products in their host organisms, which have been described as "poorly understood" to date.
[29] Many of California's Native American tribes traditionally used soaproot (genus Chlorogalum), and/or the root of various yucca species, which contain saponin, as a fish poison.
[30] The vast heterogeneity of structures underlying this class of compounds makes generalizations difficult; they're a subclass of terpenoids, oxygenated derivatives of terpene hydrocarbons.
Other kinds of molecules such as organic acids may also attach to the base, by forming esters via their carboxyl (COOH) groups.