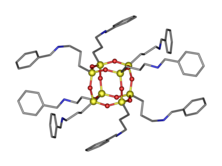
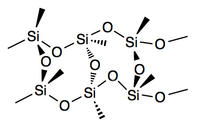
Silsesquioxane
[1] Silsesquioxanes are colorless solids that adopt cage-like or polymeric structures with Si-O-Si linkages and tetrahedral Si vertices.
In the cubic clusters with Oh symmetry the Si-O-Si angles are in the range 145–152°, being bowed out, allowing the Si centers to better adopt tetrahedral geometry.
Consequently, silsesquioxanes can be obtained directly by condensation of the corresponding silanetriols which occurs at neutral pH and works even for sterically very bulky substituents.
Bridged polysilsesquioxanes are most readily prepared from clusters that contain two or more trifunctional silyl groups attached to non-hydrolysable silicon-carbon bonds, with typical sol-gel processing.
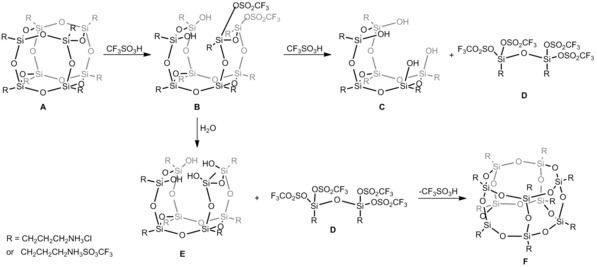
Reorganization of the siloxane cage-like core (T8 → T10) can be performed, including isolation of intermediates, and cage rearrangement achieved by using Bronsted superacid, trifluoromethanesulfonic acid (CF3SO3H).
E is prone to reaction with D and due to this, the abstraction of CF3SO−3 anion occurs and the closure frame with the spontaneous cage-rearrangement to heptahedral T10 structure F is observed.
Although, heptahedral F is less favorable energetically (MM2 data), in this case its creation is forces by the formation of a new Si4O4 moiety from much more less stable substrates D and E.[8] Polymeric silsesquioxanes have been reported, first by Brown.
Other notable silsesquioxane polymers include the soluble polymethylsilsesquioxane with high molecular weights described by Japan Synthetic Rubber.
[10] This polymer which, unlike its phenyl derivative, gels easily during the course of its synthesis, has found applications in cosmetics,[11] resins,[12] and lithography.
[13] Hierarchical organic-inorganic (hybrid) polysilsesquioxane (PSQ) materials using polyhedral oligomeric silsesquioxanes (POSS) cages as singular building blocks of the inorganic framework were synthesized by different research groups, exhibiting high specific surface area and hydrothermal stability and micro and/or mesoporosity.
[29] One of the first precursors used in light emitting application was octadimethylsiloxysilsesquioxane, which can be prepared in yields of >90% by treating tetraethoxysilane or rice hull ash with tetramethylammonium hydroxide followed by dimethylchlorosilane.
When brominated or aminated, these structures can be coupled with epoxies, aldehydes, and bromoaromatics, which enable attachment of these silsesquioxanes to π-conjugated polymers.
For chemosensor applications, silsesquioxane cages conjugated with fluorescent molecules can be directly used to detect fluoride ions under a cage-encapsulation showing a change of color under naked eyes [30] and other anions.
These desirable properties combined with the ability to readily functionalize a silsesquioxane with multiple antimicrobial groups allows for robust biocides with higher charge densities while maintaining a compact molecular structure.
In general, such silsesquioxane trisilanols form discrete dimers in the solid held together by cooperatively enhanced cyclic hydrogen bonded networks.
[39] Other partially condensed species adopt ladder structures wherein in which two long chains composed of RSiO3/2 units are connected at regular intervals by Si-O-Si bonds.
[40][41][42] Cubic metal-silsesquioxane derivatives of the core stoichiometry MSi7O12 can be prepared by treating the incomplete cage with a metal halide in the presence of a base such as triethylamine.
al synthesised a luminescent fully-condensed rare-earth-doped POSS bearing in the structure an europium ion by reaction of the open-corner heptaisobutyl trisilanol T7-POSS ((C4H9)7Si7O9(OH)3) with anhydrous EuCl3 under basic conditions.
[45] Furthermore, a combination of partially-condensed tetrasilanolphenyl POSS with terbium acetate and/or europium acetate (at different molar ratio with both metals) led to novel double-decker silsesquioxane (DDSQ) materials consisting of lanthanide-doped POSS units with intrinsic luminescent properties, in which the lanthanide ion(s) act as both structural and functional agents in the final compound.
[49] The coordination of metals to the silsesquioxane framework gives electrophilic centers that are approximately as electron-withdrawing as a trifluoromethyl group, leading to increased catalytic activity.