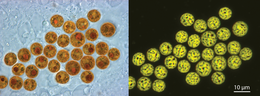
Symbiodinium
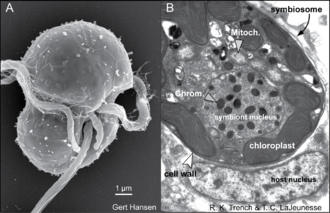
These unicellular microalgae commonly reside in the endoderm of tropical cnidarians such as corals, sea anemones, and jellyfish, where the products of their photosynthetic processing are exchanged in the host for inorganic molecules.
Cnidarians that are associated with Symbiodinium occur mostly in warm oligotrophic (nutrient-poor), marine environments where they are often the dominant constituents of benthic communities.
Under normal conditions, symbiont and host cells exchange organic and inorganic molecules that enable the growth and proliferation of both partners.
Coral reefs have economic benefits – valued at hundreds of billions of dollars each year – in the form of ornamental, subsistence and commercial fisheries, tourism and recreation, coastal protection from storms, a source of bioactive compounds for pharmaceutical development, and more.
A chief mechanism for widespread reef degradation has been stress-induced coral bleaching caused by unusually high seawater temperature.
[27][28][29] The symbiosis Symbodinium-coral could provide higher resistance to multiple stress (desiccation, high UVR) to the coral holobiont through its mycosporine-like amino acids (MAAs).
It is present in small numbers in coral globally and is common in the Andaman Sea, where the water is about 4 °C (7 °F) warmer than in other parts of the Indian Ocean.
In the Caribbean Sea in late 2005, water temperature was elevated for several months and it was found that S. trenchi, a symbiont not normally abundant, took up residence in many corals in which it had not previously been observed.
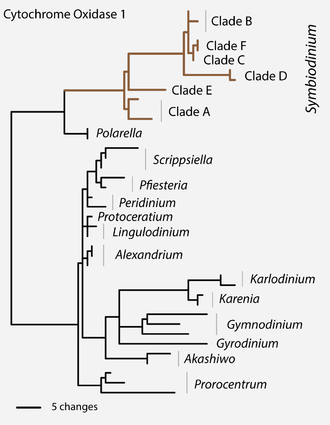
The application of this methodology helped overturn the long-held belief that (traditional understood) Symbiodinium comprised a single genus, a process which began in earnest with the morphological, physiological, and biochemical comparisons of cultured isolates.
Currently, genetic markers are exclusively used to describe ecological patterns and deduce evolutionary relationships among morphologically cryptic members of this group.
The earliest ribosomal gene sequence data indicated that Symbiodinium had lineages whose genetic divergence was similar to that seen in other dinoflagellates from different genera, families, and even orders.
The high concordance found among nuclear, mitochondrial and chloroplast DNA argues that a hierarchical phylogenetic scheme, combined with ecological and population genetic data, can unambiguously recognize and assign nomenclature to reproductively isolated lineages, i.e.
[46] Through the use of microsatellite markers, multilocus genotypes identifying a single clonal line of Symbiodinium can be resolved from samples of host tissue.
[citation needed] The earliest genetic methods for assessing Symbiodinium diversity relied on low-resolution molecular markers that separated the genus into a few evolutionarily divergent lineages, referred to as "clades".
Previous characterizations of geographic distribution and dominance have focused on the clade-level of genetic resolution, but more detailed assessments of diversity at the species level are needed.
[citation needed] The large diversity of Symbiodinium revealed by genetic analyses is distributed non-randomly and appears to comprise several guilds with distinct ecological habits.
[54] Finally, there appears to be another group of Symbiodinium that are incapable of establishing endosymbiosis yet exist in environments around the animal or associate closely with other substrates (i.e. macro-algal surfaces, sediment surface)[47][55] Symbiodinium from functional groups 2, 3, and 4 are known to exist because they culture easily, however species with these life histories are difficult to study because of their low abundance in the environment.
The use of aposymbiotic host polyps deployed as "capture vessels" and the application of molecular techniques has allowed for the detection of environmental sources of Symbiodinium.
[53][56] With these methods employed, investigators may resolve the distribution of different species on various benthic surfaces[55] and cell densities suspended in the water column.
[50] Learning more about the "private lives" of these environmental populations and their ecological function will further our knowledge about the diversity, dispersal success, and evolution among members within this large genus.
The comparison of cultured isolates under identical conditions show clear differences in morphology, size, biochemistry, gene expression, swimming behavior, growth rates, etc.
Culturing is a selective process, and many Symbiodinium isolates growing on artificial media are not typical of the species normally associated with a particular host.
Samples for genetic analysis should be acquired from the source colony in order to match the resulting culture with the identity of the dominant and ecologically relevant symbiont originally harbored by the animal.
For isolates that are in log phase growth, division rates occur every 1–3 days, with Symbiodinium cells alternating between a spherical, or coccoid, morphology and a smaller flagellated motile mastigote stage.
While several similar schemes are published that describe how each morphological state transitions to other, the most compelling life history reconstruction was deduced from light and electron microscopy and nuclear staining evidence.
[64] During asexual propagation (sometimes referred to as mitotic or vegetative growth), cells undergo a diel cycle of karyokinesis (chromosome/nuclear division) in darkness.
[64] Approaching or at the end of the photoperiod the mastigotes cease swimming, release their flagella, and undergo a rapid metamorphosis into the coccoid form.
Mucocysts (an ejectile organelle[69]) located beneath the plasmalemma are found in S. pilosum and their function is unknown, but may be involved in heterotrophic feeding.
[67] The term cyst usually refers to a dormant, metabolically quiescent stage in the life history of other dinoflagellates, initiated by several factors, including nutrient availability, temperature, and day length.
All cultured isolates (i.e. strains) are capable of phenotypic adjustment in their capacity for light harvesting (i.e. photoacclimation), such as by altering the cellular Chl.