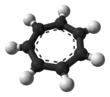
Tropylium cation
Its bromide and chloride salts[5] can be made from cycloheptatriene and bromine or phosphorus pentachloride, respectively.
The structure shown is a composite of seven resonance contributors in which each carbon atom carries part of the positive charge.
In 1891 G. Merling obtained a water-soluble bromine-containing compound from the reaction of cycloheptatriene and bromine.
Tropylium bromide was deduced to be a salt, C7H+7Br−, by Doering and Knox in 1954 by analysis of its infrared and ultraviolet spectra.
[12] Reaction with a cyclopentadienide salt of sodium or lithium yields 7-cyclopentadienylcyclohepta-1,3,5-triene:[12] When treated with oxidising agents such as chromic acid, the tropylium cation undergoes rearrangement into benzaldehyde:[12] Many metal complexes of tropylium ion are known.