Xanthan gum
Xanthan gum was discovered by Allene Rosalind Jeanes and her research team at the United States Department of Agriculture, and brought into commercial production by CP Kelco under the trade name Kelzan in the early 1960s, remaining the only manufacuturer in the US.
Xanthan gum derives its name from the species of bacteria used during the fermentation process, Xanthomonas campestris.
It is also a preferred method of thickening liquids for those with swallowing disorders, since it does not change the color or flavor of foods or beverages at typical use levels.
[6] In gluten-free baking, xanthan gum is used to give the dough or batter the stickiness that would otherwise be achieved with gluten.
[8] Furthermore, thiolated xanthan gum (see thiomers) has shown potential for drug delivery,[10][11] since by the covalent attachment of thiol groups to this polysaccharide high mucoadhesive and permeation enhancing properties can be introduced.
A teaspoon of xanthan gum weighs about 2.5 grams and brings one cup (250 ml) of water to a 1% concentration.
According to a 2017 safety review by a scientific panel of the European Food Safety Authority (EFSA), xanthan gum (European food additive number E 415) is extensively digested during intestinal fermentation, and causes no adverse effects, even at high intake amounts.
[15] The EFSA concluded that there is no safety concern for the general population when xanthan gum is consumed as a food additive.
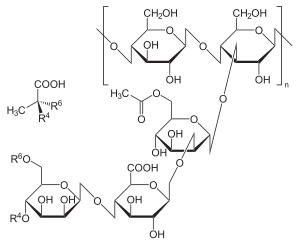
[15] It is composed of pentasaccharide repeat units, comprising glucose, mannose, and glucuronic acid in the molar ratio 2:2:1.
Whey-derived xanthan gum is commonly used in many commercial products, such as shampoos and salad dressings.
Mature repeat units are polymerized and exported in a way resembling the Wzy-dependent polysaccharide synthesis mechanism of Enterobacteriaceae.
Products of the gum gene cluster drive synthesis, polymerization, and export of the repeat unit.