Cyclohexane conformation
Therefore, the cyclohexane ring tends to assume non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape.
It is because of the symmetry of the conformations on this continuum that it is possible to satisfy all four constraints with a range of dihedral angles at (1,2,3,4).
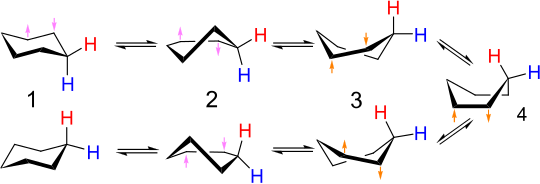
[3][4] At room temperature the molecule can easily move among these conformations, but only chair and twist-boat can be isolated in pure form, because the others are not at local energy minima.
Cyclohexane diminishes the torsional strain from eclipsing substituents through adopting a conformation with a lower number of nonplanar carbons.
Looking from directly above, the other three half-bonds will appear to point outwards towards the vertices of an equilateral triangle, so the bonds will appear to have an angle of 120° between them.
Each carbon center has one axial C–H bond (pointed alternately upwards or downwards) and one equatorial C–H bond (tilted alternately downwards or upwards), enabling each X–C–C–Y unit to adopt a staggered conformation with minimal torsional strain.
The chair geometry is often preserved when the hydrogen atoms are replaced by halogens or other simple groups.
However, when these hydrogens are substituted for a larger group, additional strain may occur due to diaxial interactions between pairs of substituents occupying the same-orientation axial position, which are typically repulsive due to steric crowding.
When one of these two four-atom chains flattens to a dihedral angle of zero, we have the half-chair conformation, at a maximum energy along the conversion path.
When the dihedral angle of this chain then becomes equal (in sign as well as magnitude) to that of the other four-atom chain, the molecule has reached the continuum of conformations, including the twist boat and the boat, where the bond angles and lengths can all be at their normal values and the energy is therefore relatively low.
Switching the signs of the two chains sequentially in this way minimizes the maximum energy state along the way (at the half-chair state) — having the dihedral angles of both four-atom chains switch sign simultaneously would mean going through a conformation of even higher energy due to angle strain at carbons 1 and 4.
For 1,2- and 1,4-disubstituted cyclohexane, a trans configuration, the diaxial conformation is effectively prevented by its high steric strain.
For 1,3-disubstituted cyclohexanes, the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups.
[10] Also, for a disubstituted cyclohexane, as well as more highly substituted molecules, the aforementioned A values are additive for each substituent.
[12] 1,3 Diaxial interactions occur when the non-hydrogen substituent on a cyclohexane occupies the axial position.
This eclipsed position increases the steric strain on the cyclohexane conformation and the confirmation will shift towards a more energetically favorable equilibrium.
[13] Gauche interactions occur when a non-hydrogen substituent on a cyclohexane occupies the equatorial position.
The cyclohexane model thus assesses steric size of functional groups on the basis of gauche interactions.
For example, a t-butyl substituent would sustain a higher energy gauche interaction as compared to a methyl group, and therefore, contribute more to the instability of the molecule as a whole.
In comparison, a staggered conformation is thus preferred; the larger groups would maintain the equatorial position and lower the energy of the entire molecule.
This preference for the equatorial position among bulkier groups lowers the energy barriers between different conformations of the ring.
When the molecule is activated, there will be a loss in entropy due to the stability of the larger substituents.
Polarity and nonpolarity are the main factors in determining how well a solvent interacts with a compound.
Cyclohexane is considered nonpolar, meaning that there is no electronegative difference between its bonds and its overall structure is symmetrical.
They exist generally follow the trends seen for cyclohexane, i.e. the chair conformer being most stable.
It was only in 1918 that Ernst Mohr [de], based on the molecular structure of diamond that had recently been solved using the then very new technique of X-ray crystallography,[18][19] was able to successfully argue that Sachse's chair was the pivotal motif.
[20][21][22][23][24][25] Derek Barton and Odd Hassel shared the 1969 Nobel Prize in Chemistry for work on the conformations of cyclohexane and various other molecules.
Cyclohexane is the most stable of the cycloalkanes, due to the stability of adapting to its chair conformer.
This specific standard signifies that cyclohexane is used in quality analysis of food and beverages, pharmaceutical release testing, and pharmaceutical method development;[26] these various methods test for purity, biosafety, and bioavailability of products.
[27] The stability of the chair conformer of cyclohexane gives the cycloalkane a versatile and important application when regarding the safety and properties of pharmaceuticals.