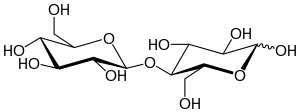
Cellobiose
The chemical structure of cellobiose is derived from the condensation of a pair of β-glucose molecules forming a β(1→4) bond.
Cellobiose has eight free alcohol (OH) groups, one acetal linkage, and one hemiacetal linkage, which give rise to strong inter- and intramolecular hydrogen bonds.
It can be obtained by enzymatic or acidic hydrolysis of cellulose and cellulose-rich materials such as cotton, jute, or paper.
[1] Cellobiose can be used as an indicator carbohydrate for Crohn's disease and malabsorption syndrome.
[2] Treatment of cellulose with acetic anhydride and sulfuric acid gives cellobiose acetoacetate, of which there is no longer a hydrogen bond donor (though it is still a hydrogen bond acceptor) and possesses aspects of being soluble in nonpolar organic solvents.