Green fluorescent protein
Scientists Roger Y. Tsien, Osamu Shimomura, and Martin Chalfie were awarded the 2008 Nobel Prize in Chemistry on 10 October 2008 for their discovery and development of the green fluorescent protein.
[9] In the 1960s and 1970s, GFP, along with the separate luminescent protein aequorin (an enzyme that catalyzes the breakdown of luciferin, releasing light), was first purified from the jellyfish Aequorea victoria and its properties studied by Osamu Shimomura.
[11] However, its utility as a tool for molecular biologists did not begin to be realized until 1992 when Douglas Prasher reported the cloning and nucleotide sequence of wtGFP in Gene.
The lab of Martin Chalfie expressed the coding sequence of wtGFP, with the first few amino acids deleted, in heterologous cells of E. coli and C. elegans, publishing the results in Science in 1994.
Further research into GFP has shown that it is resistant to detergents, proteases, guanidinium chloride (GdmCl) treatments, and drastic temperature changes.
This matched the spectral characteristics of commonly available FITC filter sets, increasing the practicality of use by the general researcher.
A 37 °C folding efficiency (F64L) point mutant to this scaffold, yielding enhanced GFP (EGFP), was discovered in 1995 by the laboratories of Thastrup[21] and Falkow.
Several additional compensatory mutations in the surrounding barrel are required to restore brightness to this modified chromophore due to the increased bulk of the indole group.
[26] Additional site-directed random mutagenesis in combination with fluorescence lifetime based screening has further stabilized the seventh β-strand resulting in a bright variant, mTurquoise2, with a quantum yield (QY) of 0.93.
[27] The red-shifted wavelength of the YFP derivatives is accomplished by the T203Y mutation and is due to π-electron stacking interactions between the substituted tyrosine residue and the chromophore.
The secondary excitation peak (480 nm) of GFP does absorb some of the blue emission of aequorin, giving the bioluminescence a more green hue.
Roger Tsien has speculated that varying hydrostatic pressure with depth may affect serine 65's ability to donate a hydrogen to the chromophore and shift the ratio of the two excitation peaks.
[37][38] Jellyfish- and coral-derived GFP-like proteins require oxygen and produce a stoichiometric amount of hydrogen peroxide upon chromophore formation.
smURFP has a large extinction coefficient (180,000 M−1 cm−1) and has a modest quantum yield (0.20), which makes it comparable biophysical brightness to eGFP and ~2-fold brighter than most red or far-red fluorescent proteins derived from coral.
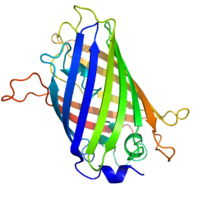
[41][42] GFP has a beta barrel structure consisting of eleven β-strands with a pleated sheet arrangement, with an alpha helix containing the covalently bonded chromophore 4-(p-hydroxybenzylidene)imidazolidin-5-one (HBI) running through the center.
[45] The tightly packed nature of the barrel excludes solvent molecules, protecting the chromophore fluorescence from quenching by water.
This protein has been shown to be an effective way to measure the toxicity levels of various chemicals including ethanol, p-formaldehyde, phenol, triclosan, and paraben.
Results from the study conducted by Song, Kim, & Seo (2016) showed that there was a decrease in both fluorescence and cellular density as pollutant levels increased.
This method is useful for studying structural and functional characteristics of the tagged protein on a macromolecular or single-molecule scale with fluorescence microscopy.
The Vertico SMI microscope using the SPDM Phymod technology uses the so-called "reversible photobleaching" effect of fluorescent dyes like GFP and its derivatives to localize them as single molecules in an optical resolution of 10 nm.
[60] GFP is considered to be a reliable reporter of gene expression in eukaryotic cells when the fluorescence is measured by flow cytometry.
[69] A novel possible use of GFP includes using it as a sensitive monitor of intracellular processes via an eGFP laser system made out of a human embryonic kidney cell line.
The first engineered living laser is made by an eGFP expressing cell inside a reflective optical cavity and hitting it with pulses of blue light.
At a certain pulse threshold, the eGFP's optical output becomes brighter and completely uniform in color of pure green with a wavelength of 516 nm.
[74] In the past, mutagenic ultra violet light (UV) has been used to illuminate living organisms (e.g., see[75]) to detect and photograph the GFP expression.
Alba, a green-fluorescent rabbit, was created by a French laboratory commissioned by Eduardo Kac using GFP for purposes of art and social commentary.
[79] The US company Yorktown Technologies markets to aquarium shops green fluorescent zebrafish (GloFish) that were initially developed to detect pollution in waterways.
[80] Green fluorescent pigs, known as Noels, were bred by a group of researchers led by Wu Shinn-Chih at the Department of Animal Science and Technology at National Taiwan University.
[82] In 2009 a South Korean team from Seoul National University bred the first transgenic beagles with fibroblast cells from sea anemones.
The dogs give off a red fluorescent light, and they are meant to allow scientists to study the genes that cause human diseases like narcolepsy and blindness.