Cysteine
The thiol side chain in cysteine enables the formation of disulfide bonds, and often participates in enzymatic reactions as a nucleophile.
L‑Cysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems.
[9] The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins.
In high-protein diets, cysteine may be partially responsible for reduced blood pressure and stroke risk.
[13] Although classified as a nonessential amino acid,[14] in rare cases, cysteine may be essential for infants, the elderly, and individuals with certain metabolic diseases or who suffer from malabsorption syndromes.
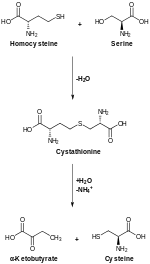
Cysteine can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available.
The majority of l-cysteine is obtained industrially by hydrolysis of animal materials, such as poultry feathers or hog hair.
[16] Indeed, food additive or cosmetic product manufactures may not legally source from human hair in the European Union.
[17][18] Some animal-originating sources of l-cysteine as a food additive contravene kosher, halal, vegan, or vegetarian diets.
[15] To avoid this problem, synthetic l-cysteine, compliant with Jewish kosher and Muslim halal laws, is also available, albeit at a higher price.
Due to the ability of thiols to undergo redox reactions, cysteine and cysteinyl residues have antioxidant properties.
[25] Beyond the iron-sulfur proteins, many other metal cofactors in enzymes are bound to the thiolate substituent of cysteinyl residues.
[27] In the translation of messenger RNA molecules to produce polypeptides, cysteine is coded for by the UGU and UGC codons.
[34] Since most cellular compartments are reducing environments, disulfide bonds are generally unstable in the cytosol with some exceptions as noted below.
Inside the cell, disulfide bridges between cysteine residues within a polypeptide support the protein's tertiary structure.
Insulin is an example of a protein with cystine crosslinking, wherein two separate peptide chains are connected by a pair of disulfide bonds.
[35][36] Similar to other later-added amino acids such as methionine, tyrosine, and tryptophan, cysteine exhibits strong nucleophilic and redox-active properties.
[41][42] In contrast, another sulfur-containing, redox-active amino acid, methionine, does not exhibit these biochemical properties and its content is relatively upregulated in mitochondrially encoded proteins.
Cysteine is a very popular target for site-directed labeling experiments to investigate biomolecular structure and dynamics.
Cysteine has been proposed as a preventive or antidote for some of the negative effects of alcohol, including liver damage and hangover.