History of electrochemistry
In recent decades, electrochemistry has become an area of current research, including research in batteries and fuel cells, preventing corrosion of metals, the use of electrochemical cells to remove refractory organics and similar contaminants in wastewater electrocoagulation and improving techniques in refining chemicals with electrolysis and electrophoresis.
In 1663, German physicist Otto von Guericke created the first electrostatic generator, which produced static electricity by applying friction.
In 1709, Francis Hauksbee at the Royal Society in London discovered that by putting a small amount of mercury in the glass of Von Guericke's generator and evacuating the air from it, it would glow whenever the ball built up a charge and his hand was touching the globe.
Between 1729 and 1736, two English scientists, Stephen Gray and Jean Desaguliers, performed a series of experiments which showed that a cork or other object as far away as 800 or 900 feet (245–275 m) could be electrified by connecting it via a charged glass tube to materials such as metal wires or hempen string.
Nollet's theory at first gained wide acceptance, but met resistance in 1752 with the translation of Franklin's Experiments and Observations on Electricity into French.
The general belief at the time was that electricity was faster than sound, but no accurate test had been devised to measure the velocity of a current.
Watson, in the fields north of London, laid out a line of wire supported by dry sticks and silk which ran for 12,276 feet (3.7 km).
Coulomb wrote seven important works on electricity and magnetism which he submitted to the Académie des Sciences between 1785 and 1791, in which he reported having developed a theory of attraction and repulsion between charged bodies, and went on to search for perfect conductors and dielectrics.
Galvani's scientific colleagues generally accepted his views, but Alessandro Volta, the outstanding professor of physics at the University of Pavia, was not convinced by the analogy between muscles and Leyden jars.
Deciding that the frogs' legs used in Galvani's experiments served only as an electroscope, he held that the contact of dissimilar metals was the true source of stimulation.
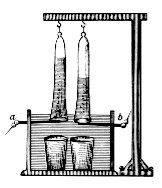
In 1800, English chemists William Nicholson and Johann Wilhelm Ritter succeeded in separating water into hydrogen and oxygen by electrolysis.
The passage of current caused the acid to decompose chemically, and the message was read by observing at which of the terminals the bubbles of gas appeared.
Hans Christian Ørsted's discovery of the magnetic effect of electric currents in 1820 was immediately recognised as an important advance, although he left further work on electromagnetism to others.
During the 1820s, Robert Hare developed the Deflagrator, a form of voltaic battery having large plates used for producing rapid and powerful combustion.
In 1821, the Estonian-German physicist, Thomas Johann Seebeck, demonstrated the electrical potential in the juncture points of two dissimilar metals when there is a temperature difference between the joints.
In 1827 German scientist Georg Ohm expressed his law in his famous book Die galvanische Kette, mathematisch bearbeitet (The Galvanic Circuit Investigated Mathematically) in which he gave his complete theory of electricity.
Solar cell technology dates to 1839 when Becquerel observed that shining light on an electrode submerged in a conductive solution would create an electric current.
John Daniell began experiments in 1835 in an attempt to improve the voltaic battery with its problems of being unsteady and a weak source of electric current.
Eventually the term fuel cell was coined in 1889 by Ludwig Mond and Charles Langer, who attempted to build the first practical device using air and industrial coal gas.
Grove's nitric acid cell was the favourite battery of the early American telegraph (1840–1860), because it offered strong current output.
Svante August Arrhenius published his thesis in 1884, Recherches sur la conductibilité galvanique des électrolytes (Investigations on the galvanic conductivity of electrolytes).
From the results of his experiments, the author concluded that electrolytes, when dissolved in water, become to varying degrees split or dissociated into positive and negative ions.
Ostwald is especially known for his contributions to the field of electrochemistry, including important studies of the electrical conductivity and electrolytic dissociation of organic acids.
Nernst's early studies in electrochemistry were inspired by Arrhenius' dissociation theory which first recognised the importance of ions in solution.
In 1909, Robert Andrews Millikan began a series of experiments to determine the electric charge carried by a single electron.
He obtained more precise results in 1910 with his famous oil-drop experiment in which he replaced water (which tended to evaporate too quickly) with oil.
Jaroslav Heyrovský, a Nobel laureate, eliminated the tedious weighing required by previous analytical techniques, which used the differential precipitation of mercury by measuring drop-time.
In 1923, Johannes Nicolaus Brønsted and Thomas Martin Lowry published essentially the same theory about how acids and bases behave using electrochemical basis.
The International Society of Electrochemistry (ISE) was founded in 1949, and some years later the first sophisticated electrophoretic apparatus was developed in 1937 by Arne Tiselius, who was awarded the 1948 Nobel prize for his work in protein electrophoresis.
Electrophoresis became widely developed in the 1940s and 1950s when the technique was applied to molecules ranging from the largest proteins to amino acids and even inorganic ions.