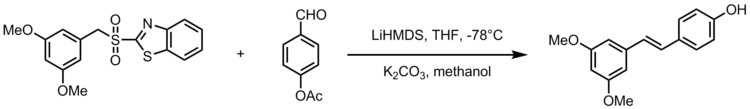Julia olefination
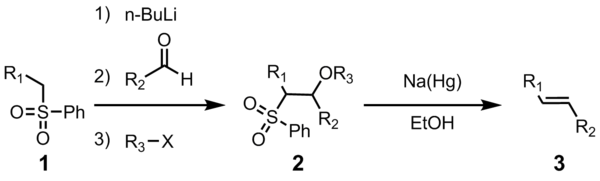
In 1973, Marc Julia and Jean-Marc Paris reported a novel olefin synthesis in which β-acyloxysulfones were reductively eliminated to the corresponding di-, tri-, or tetrasubstituted alkenes.
Unlike the phenyl sulfones, this alkoxide intermediate (2) is more reactive and will undergo a Smiles rearrangement[15] to give the sulfinate salt (4).
The sulfinate salt (4) will spontaneously eliminate sulfur dioxide and lithium benzothiazolone (5) producing the desired alkene (6).
Since the benzothiazole variation of the Julia olefination does not involve equilibrating intermediates, the stereochemical outcome is a result of the stereochemistry of the initial carbonyl addition.
The high E-selectivity of the Julia–Kocienski olefination is the result of kinetically controlled diastereoselective addition of metalated 1-phenyl-1H-tetrazol-5-yl (PT) sulfones to nonconjugated aldehydes.
[17] In one adaptation,[18] with t-butyltetrazoylmethyl sulfone the reaction conditions are either sodium bis(trimethylsilyl)amide at −70 °C in tetrahydrofuran or caesium carbonate at +70 °C.
Compared to the Wittig, Wittig-Horner, Perkin, or transition-metal-catalyzed reactions to synthesize pterostilebene, the Julia olefination offers a simple, economical alternative method for preparation of pterostilbene.
[20][21] One adaptation of the Julia-Kocienski olefination gives the synthesis of the stilbenoid resveratrol, a natural compound found in common foods like grapes, wines and nuts.
The Julia-Kocienski olefination serves as a powerful reaction in the synthesis of resveratrol analogues with 3,5-bis(trifluoromethyl)phenyl sulfones.
[22] In the asymmetric total synthesis of (−)-callystatin A by Amos Smith, two separate Julia olefinations were used to append two E-alkene moieties.