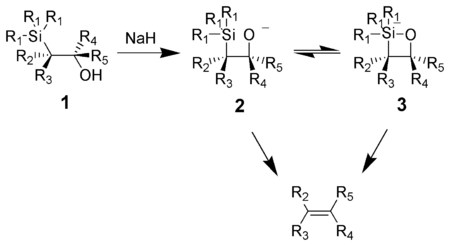
Peterson olefination
The action of base upon a β-hydroxysilane (1) results in a concerted syn elimination of (2) or (3) to form the desired alkene.
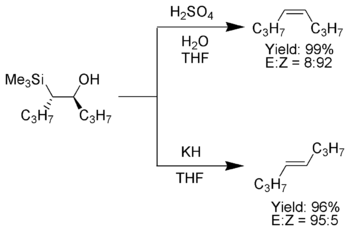
The treatment of the β-hydroxysilane (1) with acid results in protonation and an anti elimination to form the desired alkene.
When the α-silyl carbanion contains only alkyl, hydrogen, or electron-donating substituents, the stereochemical outcome of the Peterson olefination can be controlled,[7] because at low temperature the elimination is slow and the intermediate β-hydroxysilane can be isolated.
Additionally, elimination using sodium or potassium hydride may not be feasible due to incompatible functional groups.
[9] Corey and co-workers developed a method (sometimes dubbed the Corey-Peterson olefination[10]) using a silylated imine to yield an α,β-unsaturated aldehyde from a carbonyl compound in one step.