Shapiro reaction
In a prelude to the actual Shapiro reaction, a ketone or an aldehyde (1) is reacted with p-toluenesulfonylhydrazide[6](2) to form a p-toluenesulfonylhydrazone (or tosylhydrazone) which is a hydrazone (3).
This diazonium anion is then lost as molecular nitrogen resulting in a vinyllithium species (7), which can then be reacted with various electrophiles, including simple neutralization with water or an acid (8).
To combat this problem, Yamamoto and coworkers developed an efficient stereoselective and regioselective route to alkenes using a combination of ketone phenylaziridinylhydrazones as arenesulfonylhydrazone equivalents with a catalytic amount of lithium amides.
The high stereoselectivity is obtained by the preferential abstraction of the α-methylene hydrogen syn to the phenylaziridine, and is also accounted for by the internal chelation of the lithiated intermediated.
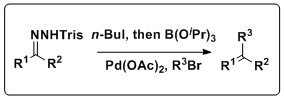
Keay and coworkers have developed methodology that combines these reactions in a one pot process that does not require the isolation of the boronic acid, a setback of the traditional Suzuki coupling.
K. Mori and coworkers wanted to determine the absolute configuration of the phytocassane group of a class of natural products called phytoalexins.