Phospholipase D
[7] Phospholipase D is an important player in many physiological processes, including membrane trafficking, cytoskeletal reorganization, receptor-mediated endocytosis, exocytosis, and cell migration.
[11] More than half a century of biochemical studies have implicated phospholipase D and PA activity in a wide range of physiological processes and diseases, including inflammation, diabetes, phagocytosis, neuronal & cardiac signaling, and oncogenesis.
[12] Strictly speaking, phospholipase D is a transphosphatidylase: it mediates the exchange of polar headgroups covalently attached to membrane-bound lipids.
Utilizing water as a nucleophile, this enzyme catalyzes the cleavage of the phosphodiester bond in structural phospholipids such as phosphatidylcholine and phosphatidylethanolamine.
[14] In addition, some members of the PLD superfamily may employ primary alcohols such as ethanol or 1-butanol in the cleavage of the phospholipid, effectively catalyzing the exchange the polar lipid headgroup.
[6][11] Other members of this family are able hydrolyze other phospholipid substrates, such as cardiolipin, or even the phosphodiester bond constituting the backbone of DNA.
Additionally, PA can be converted into a number of other lipids, such as lysophosphatidic acid (lyso-PA) or diacylglycerol, signal molecules which have a multitude of effects on downstream cellular pathways.
[11] PA and its lipid derivatives are implicated in myriad processes that include intracellular vesicle trafficking, endocytosis, exocytosis, actin cytoskeleton dynamics, cell proliferation differentiation, and migration.
PA is extremely short lived and is rapidly hydrolysed by the enzyme phosphatidate phosphatase to form diacylglycerol (DAG).
Thus functional saturated/monounsaturated PA can be degraded by hydrolysing it to form non-functional saturated/monounsaturated DAG while functional polyunsaturated DAG can be degraded by converting it into non-functional polyunsaturated PA.[16][17][18] A lysophospholipase D called autotaxin was recently identified as having an important role in cell-proliferation through its product, lysophosphatidic acid (LPA).
Plant and animal PLDs have a consistent molecular structure, characterized by sites of catalysis surrounded by an assortment of regulatory sequences.
Each of these appears to possess a domain duplication which is apparent by the presence of two HKD motifs containing well-conserved histidine, lysine, and asparagine residues which may contribute to the active site aspartic acid.
Functioning as nucleophiles, the constituent imidazole moieties of the histidines form transient covalent bonds with the phospholipid, producing a short-lived intermediate that can be easily hydrolyzed by water in a subsequent step.
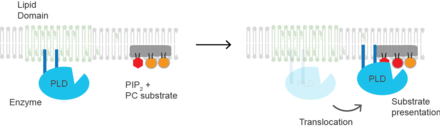
[25] The protein may also undergo a conformational change upon PIP2 binding, but this has not been shown experimentally and would constitute a mechanism of activation distinct from substrate presentation.
Two major isoforms of phospholipase D has been identified in mammalian cells: PLD1 and PLD2 (53% sequence homology),[27] each encoded by distinct genes.
[6] In plants and animals, this binding site is characterized by the presence of a conserved sequence of basic and aromatic amino acids.
Phospholipase D is a regulator of several critical cellular processes, including vesicle transport, endocytosis, exocytosis, cell migration, and mitosis.
[8] Dysregulation of these processes is commonplace in carcinogenesis,[8] and in turn, abnormalities in PLD expression have been implicated in the progression of several types cancer.
[7] One potential hypothesis casts a critical role for phospholipase D in the activation of mTOR, a suppressor of cancer cell apoptosis.
[7] The ability of PLD to suppress apoptosis in cells with elevated tyrosine kinase activity makes it a candidate oncogene in cancers where such expression is typical.
[8] Phospholipase D may also play an important pathophysiological role in the progression of neurodegenerative diseases, primarily through its capacity as a signal transducer in indispensable cellular processes like cytoskeletal reorganization and vesicle trafficking.
[7] Abnormal PLD activity has also been suspected in Alzheimer's disease, where it has been observed to interact with presenilin 1 (PS-1), the principal component of the γ-secretase complex responsible for the enzymatic cleavage of amyloid precursor protein (APP).