Hsp90
It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs.
[3] As their name implies, heat shock proteins protect cells when stressed by elevated temperatures.
[8] The α- and the β-forms are thought to be the result of a gene duplication event that occurred millions of years ago.
[18][25] The C-terminal domain possesses an alternative ATP-binding site, which becomes accessible when the N-terminal Bergerat pocket is occupied.
[26][27] At the very C-terminal end of the protein is the tetratricopeptide repeat (TPR) motif recognition site, the conserved MEEVD pentapeptide, that is responsible for the interaction with co-factors such as the immunophilins FKBP51 and FKBP52, the stress induced phosphoprotein 1 (Sti1/Hop), cyclophilin-40, PP5, Tom70, and many more.
[31] Antitumor drugs targeting this section of Hsp90 include the antibiotics geldanamycin,[11][32] herbimycin, radicicol, deguelin,[33] derrubone,[34] macbecin,[35] and beta-lactams.
Thus, ATP hydrolysis drives what is commonly referred to as a “pincer-type” conformational change in the protein binding site.
[39] The ability of Hsp90 to clamp onto proteins allows it to perform several functions including assisting folding, preventing aggregation, and facilitating transport.
[40] Furthermore, Hsp90 has been shown to suppress the aggregation of a wide range of "client" or "substrate" proteins and hence acts as a general protective chaperone.
[44] Eukaryotic proteins that are no longer needed or are misfolded or otherwise damaged are usually marked for destruction by the polyubiquitation pathway.
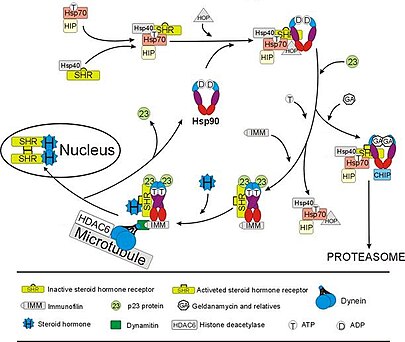
[50][51] In the absence of the steroid hormone cortisol, GR resides in the cytosol complexed with several chaperone proteins including Hsp90 (see figure to the right).
A second role of Hsp90 is to bind immunophilins (e.g., FKBP52) that attach the GR complex to the dynein protein trafficking pathway, which translocates the activated receptor from the cytoplasm into the nucleus.
Hsp90 is also required for the proper functioning of several other steroid receptors, including those responsible for the binding of aldosterone,[53] androgen,[54] estrogen,[55] and progesterone.
[59][15][60] Interestingly, the disruption of HSP90 with nano-therapeutics has been implicated in targeting drug-induced resistance and relieves the suppression of Natural Killer (NK) immune cells in breast cancer.
[62] Hsp90 is also required for induction of vascular endothelial growth factor (VEGF) and nitric oxide synthase (NOS).
[24] Both are important for de novo angiogenesis that is required for tumour growth beyond the limit of diffusion distance of oxygen in tissues.
[63] Together with its co-chaperones, Hsp90 modulates tumour cell apoptosis "mediated through effects on AKT,[23] tumor necrosis factor receptors (TNFR) and nuclear factor-κB (NF-κB) function.".
[64] Also, Hsp90 participates in many key processes in oncogenesis such as self-sufficiency in growth signals, stabilization of mutant proteins, angiogenesis, and metastasis.
Hsp90 plays apparently conflicting roles in the cell, as it is essential for both the creation and the maintenance as well as the destruction of proteins.
[66] Prediction and validation of the immunodominant epitope/s of HSP90 beta protein has been demonstrated using sera from infertile women having anti-HSP90 autoantibodies.
A polyclonal antibody generated to the immunodominant epitope- EP6 confirms similar biochemical and cellular immunoreactivity as seen with the patients' sera with anti-HSP90 autoantibodies.
This inference is supported by the fact that the duplication is found in Giardia lamblia, one of the earliest branching eukaryotic species.