Iron compounds
Iron shows the characteristic chemical properties of the transition metals, namely the ability to form variable oxidation states differing by steps of one and a very large coordination and organometallic chemistry: indeed, it was the discovery of an iron compound, ferrocene, that revolutionalized the latter field in the 1950s.
[1] Iron is sometimes considered as a prototype for the entire block of transition metals, due to its abundance and the immense role it has played in the technological progress of humanity.
[11] Iron is by far the most reactive element in its group; it is pyrophoric when finely divided and dissolves easily in dilute acids, giving Fe2+.
The ferrous halides typically arise from treating iron metal with the corresponding hydrohalic acid to give the corresponding hydrated salts.
[13] Ferric iodide is an exception, being thermodynamically unstable due to the oxidizing power of Fe3+ and the high reducing power of I−:[13] Ferric iodide, a black solid, is not stable in ordinary conditions, but can be prepared through the reaction of iron pentacarbonyl with iodine and carbon monoxide in the presence of hexane and light at the temperature of −20 °C, with oxygen and water excluded.
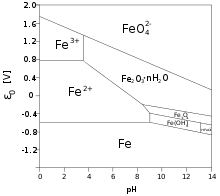
[14][15] The standard reduction potentials in acidic aqueous solution for some common iron ions are given below:[10] The red-purple tetrahedral ferrate(VI) anion is such a strong oxidizing agent that it oxidizes nitrogen and ammonia at room temperature, and even water itself in acidic or neutral solutions:[13] The Fe3+ ion has a large simple cationic chemistry, although the pale-violet hexaquo ion [Fe(H2O)6]3+ is very readily hydrolyzed when pH increases above 0 as follows:[16] As pH rises above 0 the above yellow hydrolyzed species form and as it rises above 2–3, reddish-brown hydrous iron(III) oxide precipitates out of solution.
Thus, all the above complexes are rather strongly colored, with the single exception of the hexaquo ion – and even that has a spectrum dominated by charge transfer in the near ultraviolet region.
[16] On the other hand, the pale green iron(II) hexaquo ion [Fe(H2O)6]2+ does not undergo appreciable hydrolysis.
For example, the trans-chlorohydridobis(bis-1,2-(diphenylphosphino)ethane)iron(II) complex is used as a starting material for compounds with the Fe(dppe)2 moiety.
[16] Potassium ferrioxalate is used in chemical actinometry and along with its sodium salt undergoes photoreduction applied in old-style photographic processes.
Chloro complexes are less stable and favor tetrahedral coordination as in [FeCl4]−; [FeBr4]− and [FeI4]− are reduced easily to iron(II).
Like manganese(II), most iron(III) complexes are high-spin, the exceptions being those with ligands that are high in the spectrochemical series such as cyanide.
The cyanide ligands may easily be detached in [Fe(CN)6]3−, and hence this complex is poisonous, unlike the iron(II) complex [Fe(CN)6]4− found in Prussian blue,[16] which does not release hydrogen cyanide except when dilute acids are added.
Prussian blue or "ferric ferrocyanide", Fe4[Fe(CN)6]3, is an old and well-known iron-cyanide complex, extensively used as pigment and in several other applications.
[23] A landmark in this field was the discovery in 1951 of the remarkably stable sandwich compound ferrocene Fe(C5H5)2, by Pauson and Kealy[24] and independently by Miller and colleagues,[25] whose surprising molecular structure was determined only a year later by Woodward and Wilkinson[26] and Fischer.








carbonyl
