Chemistry
[7] For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties of the soil on the Moon (cosmochemistry), how medications work (pharmacology), and how to collect DNA evidence at a crime scene (forensics).
[8] It has evolved, and now chemistry encompasses various areas of specialisation, or subdisciplines, that continue to increase in number and interrelate to create further interdisciplinary fields of study.
Alchemy is often associated with the quest to turn lead or other base metals into gold, though alchemists were also interested in many of the questions of modern chemistry.
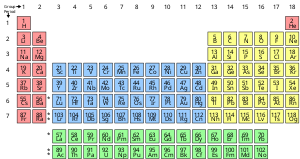
[13] Traditional chemistry starts with the study of elementary particles, atoms, molecules,[14] substances, metals, crystals and other aggregates of matter.
Charged polyatomic collections residing in solids (for example, common sulfate or nitrate ions) are generally not considered "molecules" in chemistry.
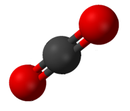
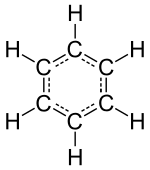
Identifiable molecules compose familiar substances such as water, air, and many organic compounds like alcohol, sugar, gasoline, and the various pharmaceuticals.
These other types of substances, such as ionic compounds and network solids, are organized in such a way as to lack the existence of identifiable molecules per se.
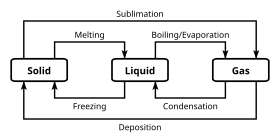
A phase is a set of states of a chemical system that have similar bulk structural properties, over a range of conditions, such as pressure or temperature.
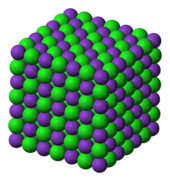
The ions are held together due to electrostatic attraction, and that compound sodium chloride (NaCl), or common table salt, is formed.
Despite being unsuccessful in explaining the nature of matter and its transformations, alchemists set the stage for modern chemistry by performing experiments and recording the results.
Robert Boyle, although skeptical of elements and convinced of alchemy, played a key part in elevating the "sacred art" as an independent, fundamental and philosophical discipline in his work The Sceptical Chymist (1661).
[35] While both alchemy and chemistry are concerned with matter and its transformations, the crucial difference was given by the scientific method that chemists employed in their work.
Chemistry, as a body of knowledge distinct from alchemy, became an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena.
The term "chymistry", in the view of noted scientist Robert Boyle in 1661, meant the subject of the material principles of mixed bodies.
[37] In 1663, the chemist Christopher Glaser described "chymistry" as a scientific art, by which one learns to dissolve bodies, and draw from them the different substances on their composition, and how to unite them again, and exalt them to a higher perfection.
[40] This definition further evolved until, in 1947, it came to mean the science of substances: their structure, their properties, and the reactions that change them into other substances—a characterization accepted by Linus Pauling.
[42] Early civilizations, such as the Egyptians,[43] Babylonians, and Indians,[44] amassed practical knowledge concerning the arts of metallurgy, pottery and dyes, but did not develop a systematic theory.
[49] In the Hellenistic world the art of alchemy first proliferated, mingling magic and occultism into the study of natural substances with the ultimate goal of transmuting elements into gold and discovering the elixir of eternal life.
[50] Work, particularly the development of distillation, continued in the early Byzantine period with the most famous practitioner being the 4th century Greek-Egyptian Zosimos of Panopolis.
[51] Alchemy continued to be developed and practised throughout the Arab world after the Muslim conquests,[52] and from there, and from the Byzantine remnants,[53] diffused into medieval and Renaissance Europe through Latin translations.
Improvements of the refining of ores and their extractions to smelt metals was widely used source of information for early chemists in the 16th century, among them Georg Agricola (1494–1555), who published his major work De re metallica in 1556.
[35] He formulated Boyle's law, rejected the classical "four elements" and proposed a mechanistic alternative of atoms and chemical reactions that could be subject to rigorous experiment.
The Scottish chemist Joseph Black and the Flemish Jan Baptist van Helmont discovered carbon dioxide, or what Black called 'fixed air' in 1754; Henry Cavendish discovered hydrogen and elucidated its properties and Joseph Priestley and, independently, Carl Wilhelm Scheele isolated pure oxygen.
Lavoisier did more than any other to establish the new science on proper theoretical footing, by elucidating the principle of conservation of mass and developing a new system of chemical nomenclature used to this day.
The development of the electrochemical theory of chemical combinations occurred in the early 19th century as the result of the work of two scientists in particular, Jöns Jacob Berzelius and Humphry Davy, made possible by the prior invention of the voltaic pile by Alessandro Volta.
[72] Other crucial 19th century advances were; an understanding of valence bonding (Edward Frankland in 1852) and the application of thermodynamics to chemistry (J. W. Gibbs and Svante Arrhenius in the 1870s).
At the turn of the twentieth century the theoretical underpinnings of chemistry were finally understood due to a series of remarkable discoveries that succeeded in probing and discovering the very nature of the internal structure of atoms.
In 1897, J.J. Thomson of the University of Cambridge discovered the electron and soon after the French scientist Becquerel as well as the couple Pierre and Marie Curie investigated the phenomenon of radioactivity.
In a series of pioneering scattering experiments Ernest Rutherford at the University of Manchester discovered the internal structure of the atom and the existence of the proton, classified and explained the different types of radioactivity and successfully transmuted the first element by bombarding nitrogen with alpha particles.
The electronic theory of chemical bonds and molecular orbitals was developed by the American scientists Linus Pauling and Gilbert N. Lewis.




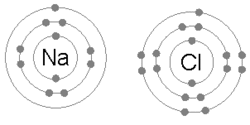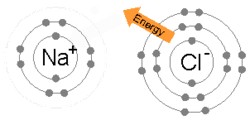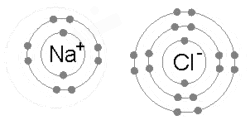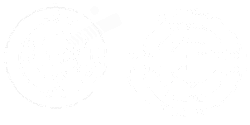









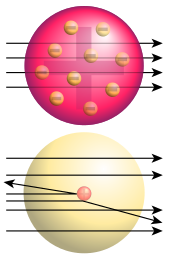
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated charge .




