Reactive oxygen species
Most often however, ROS are discussed in a biological context, ranging from their effects on aging and their role in causing dangerous genetic mutations.
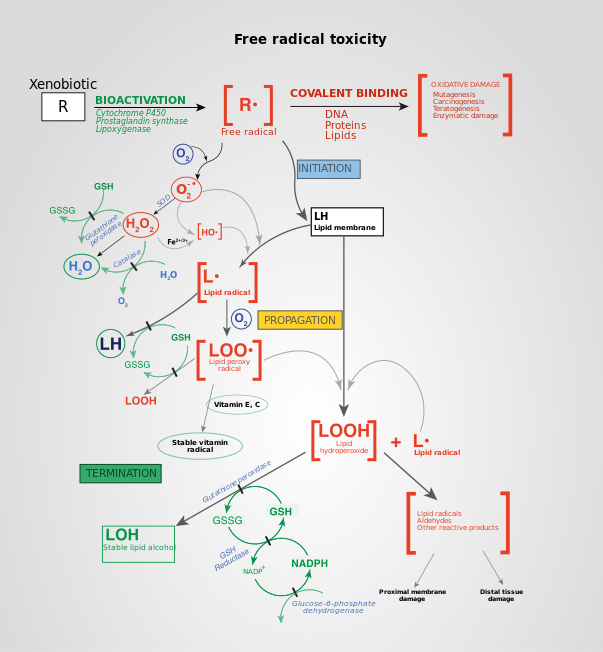
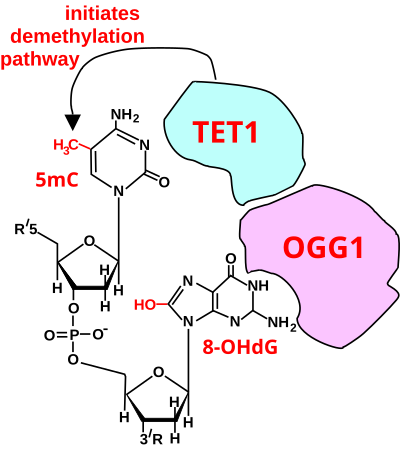
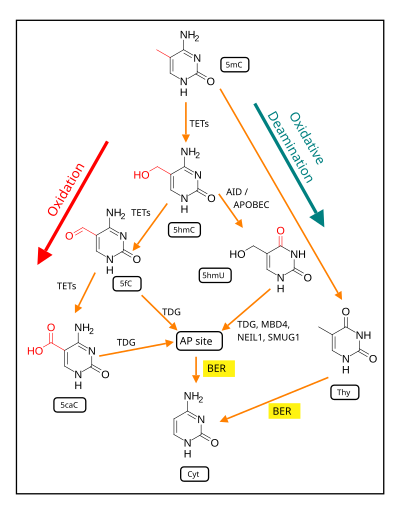
[12] However, ROS can cause irreversible damage to DNA as they oxidize and modify some cellular components and prevent them from performing their original functions.
In normal conditions, the oxygen is reduced to produce water; however, in about 0.1–2% of electrons passing through the chain (this number derives from studies in isolated mitochondria, though the exact rate in live organisms is yet to be fully agreed upon), oxygen is instead prematurely and incompletely reduced to give the superoxide radical (•O−2), most well documented for Complex I and Complex III.
[23] Another source of ROS production in animal cells is the electron transfer reactions catalyzed by the mitochondrial P450 systems in steroidogenic tissues.
To cope with this natural source of ROS, the steroidogenic tissues, ovary and testis, have a large concentration of antioxidants such as vitamin C (ascorbate) and β-carotene and anti-oxidant enzymes.
[28][29] In chloroplasts, the carboxylation and oxygenation reactions catalyzed by rubisco ensure that the functioning of the electron transport chain (ETC) occurs in an environment rich in O2.
[30] In cases where there is an ETC overload, part of the electron flow is diverted from ferredoxin to O2, forming the superoxide free radical (by the Mehler reaction).
[31] The formation of ROS can be stimulated by a variety of agents such as pollutants, heavy metals,[19] tobacco, smoke, drugs, xenobiotics, microplastics, or radiation.
In plants, in addition to the action of dry abiotic factors, high temperature, interaction with other living beings can influence the production of ROS.
[citation needed] In plants, the production of ROS occurs during events of abiotic stress that lead to a reduction or interruption of metabolic activity.
However, the second phase of ROS accumulation is associated only with infection by non-virulent pathogens and is an induced response dependent on increased mRNA transcription encoding enzymes.
[citation needed] The SOD-catalysed dismutation of superoxide may be written with the following half-reactions: where M = Cu (n = 1); Mn (n = 2); Fe (n = 2); Ni (n = 2).
[19] These include not only roles in apoptosis (programmed cell death) but also positive effects such as the induction of host defence[36][37] genes and mobilization of ion transporters.
[citation needed] Reactive oxygen species are implicated in cellular activity to a variety of inflammatory responses including cardiovascular disease.
They may also be involved in hearing impairment via cochlear damage induced by elevated sound levels, in ototoxicity of drugs such as cisplatin, and in congenital deafness in both animals and humans.
[38] When a plant recognizes an attacking pathogen, one of the first induced reactions is to rapidly produce superoxide (O−2) or hydrogen peroxide (H2O2) to strengthen the cell wall.
[28] To highlight the importance of this defense, individuals with chronic granulomatous disease who have deficiencies in generating ROS, are highly susceptible to infection by a broad range of microbes including Salmonella enterica, Staphylococcus aureus, Serratia marcescens, and Aspergillus spp.
Studies on the homeostasis of the Drosophila melanogaster's intestines have shown the production of ROS as a key component of the immune response in the gut of the fly.
ROS acts both as a bactericide, damaging the bacterial DNA, RNA and proteins, as well as a signalling molecule that induces repair mechanisms of the epithelium.
The tight regulation of DUOX avoids excessive production of ROS and facilitates differentiation between benign and damage-inducing microorganisms in the gut.
[45] In aerobic organisms the energy needed to fuel biological functions is produced in the mitochondria via the electron transport chain.
But under oxidative stress conditions, excessive ROS can damage cellular proteins, lipids and DNA, leading to fatal lesions in the cell that contribute to carcinogenesis.
On the other hand, a high level of ROS can suppress tumor growth through the sustained activation of cell-cycle inhibitor[56][57] and induction of cell death as well as senescence by damaging macromolecules.
As a result, production of NADPH is greatly enhanced, which functions as a cofactor to provide reducing power in many enzymatic reactions for macromolecular biosynthesis and at the same time rescuing the cells from excessive ROS produced during rapid proliferation.
[71] ROS can also induce cell death through autophagy, which is a self-catabolic process involving sequestration of cytoplasmic contents (exhausted or damaged organelles and protein aggregates) for degradation in lysosomes.
When this type of cell death occurs, an increase or loss of control of autophagy regulating genes is commonly co-observed.
[75] Both in vitro and in vivo, ROS have been shown to induce transcription factors and modulate signaling molecules involved in angiogenesis (MMP, VEGF) and metastasis (upregulation of AP-1, CXCR4, AKT and downregulation of PTEN).
[61] ROS induces chronic inflammation by the induction of COX-2, inflammatory cytokines (TNFα, interleukin 1 (IL-1), IL-6), chemokines (IL-8, CXCR4) and pro-inflammatory transcription factors (NF-κB).
Radiotherapy uses X-rays, γ-rays as well as heavy particle radiation such as protons and neutrons to induce ROS-mediated cell death and mitotic failure.
Combinations of ROS-generating drugs with pharmaceuticals that can break the redox adaptation could be a better strategy for enhancing cancer cell cytotoxicity.
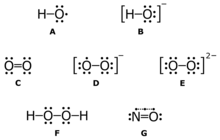
A : hydroxyl radical ( HO • );
B : hydroxide ion ( HO − );
C : singlet oxygen ( 1 O 2 );
D : superoxide anion ( O 2 •− );
E : peroxide ion ( O 2− 2 );
F : hydrogen peroxide ( H 2 O 2 );
G : nitric oxide ( NO • )